Quantitative analysis of multivesicular bodies (MVBs) in the hypoglossal nerve: evidence that neurotrophic factors do not use MVBs for retrograde axonal transport
- PMID: 19363811
- PMCID: PMC2861426
- DOI: 10.1002/cne.22047
Quantitative analysis of multivesicular bodies (MVBs) in the hypoglossal nerve: evidence that neurotrophic factors do not use MVBs for retrograde axonal transport
Abstract
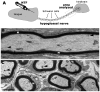
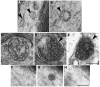
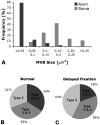
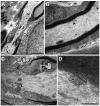
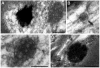
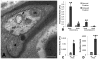
Multivesicular bodies (MVBs) are defined by multiple internal vesicles enclosed within an outer, limiting membrane. MVBs have previously been quantified in neuronal cell bodies and in dendrites, but their frequencies and significance in axons are controversial. Despite lack of conclusive evidence, it is widely believed that MVBs are the primary organelle that carries neurotrophic factors in axons. Reliable information about axonal MVBs under physiological and pathological conditions is needed for a realistic assessment of their functional roles in neurons. We provide a quantitative ultrastructural analysis of MVBs in the normal postnatal rat hypoglossal nerve and under a variety of experimental conditions. MVBs were about 50 times less frequent in axons than in neuronal cell bodies or dendrites. Five distinct types of MVBs were distinguished in axons, based on MVB size, electron density, and size of internal vesicles. Although target manipulations did not significantly change MVBs in axons, dystrophic conditions such as delayed fixation substantially increased the number of axonal MVBs. Radiolabeled brain- and glial-cell derived neurotrophic factors (BDNF and GDNF) injected into the tongue did not accumulate during retrograde axonal transport in MVBs, as determined by quantitative ultrastructural autoradiography, and confirmed by analysis of quantum dot-labeled BDNF. We conclude that for axonal transport, neurotrophic factors utilize small vesicles or endosomes that can be inconspicuous at transmission electron microscopic resolution, rather than MVBs. Previous reports of axonal MVBs may be based, in part, on artificial generation of such organelles in axons due to dystrophic conditions.
Figures

Similar articles
-
Synaptic targeting of retrogradely transported trophic factors in motoneurons: comparison of glial cell line-derived neurotrophic factor, brain-derived neurotrophic factor, and cardiotrophin-1 with tetanus toxin.J Neurosci. 2005 Jan 19;25(3):539-49. doi: 10.1523/JNEUROSCI.4322-04.2005. J Neurosci. 2005. PMID: 15659589 Free PMC article.
-
Anterograde axonal transport of glial cell line-derived neurotrophic factor and its receptors in rat hypoglossal nerve.Neuroscience. 2000;97(3):575-80. doi: 10.1016/s0306-4522(00)00079-8. Neuroscience. 2000. PMID: 10828539
-
Multivesicular bodies in neurons: distribution, protein content, and trafficking functions.Prog Neurobiol. 2011 Mar;93(3):313-40. doi: 10.1016/j.pneurobio.2011.01.003. Epub 2011 Jan 7. Prog Neurobiol. 2011. PMID: 21216273 Free PMC article. Review.
-
Anterograde axonal transport of internalized GDNF in sensory and motor neurons.Neuroreport. 2002 Apr 16;13(5):659-64. doi: 10.1097/00001756-200204160-00025. Neuroreport. 2002. PMID: 11973466
-
Retrograde signaling via axonal transport through signaling endosomes.J Pharmacol Sci. 2019 Oct;141(2):91-96. doi: 10.1016/j.jphs.2019.10.001. Epub 2019 Oct 17. J Pharmacol Sci. 2019. PMID: 31679963 Review.
Cited by
-
The emerging role of exosomes in mental disorders.Transl Psychiatry. 2019 Mar 28;9(1):122. doi: 10.1038/s41398-019-0459-9. Transl Psychiatry. 2019. PMID: 30923321 Free PMC article. Review.
-
Stress-Related Roles of Exosomes and Exosomal miRNAs in Common Neuropsychiatric Disorders.Int J Mol Sci. 2024 Jul 29;25(15):8256. doi: 10.3390/ijms25158256. Int J Mol Sci. 2024. PMID: 39125827 Free PMC article. Review.
-
Mutations in ap1b1 cause mistargeting of the Na(+)/K(+)-ATPase pump in sensory hair cells.PLoS One. 2013 Apr 12;8(4):e60866. doi: 10.1371/journal.pone.0060866. Print 2013. PLoS One. 2013. PMID: 23593334 Free PMC article.
-
Sunday driver interacts with two distinct classes of axonal organelles.J Biol Chem. 2009 Dec 11;284(50):34628-39. doi: 10.1074/jbc.M109.035022. Epub 2009 Sep 29. J Biol Chem. 2009. PMID: 19801628 Free PMC article.
-
The identification of novel small extracellular vesicle (sEV) production modulators using luciferase-based sEV quantification method.J Extracell Biol. 2022 Sep 27;1(9):e62. doi: 10.1002/jex2.62. eCollection 2022 Sep. J Extracell Biol. 2022. PMID: 38938770 Free PMC article.
References
-
- Altick AL, Baryshnikova LM, Damke H, von Bartheld CS. Retrograde axonal transport of neurotrophic factors in vivo. Int J Dev Neurosci. 2008;26:870–871.
-
- Armstrong R, Toews AD, Morell P. Axonal transport through nodes of Ranvier. Brain Res. 1987;412:196–199. - PubMed
-
- Berthold CH, Fabricius C, Rydmark M, Andersen B. Axoplasmic organelles at nodes of Ranvier. I. Occurrence and distribution in large myelinated spinal root axons of the adult cat. J Neurocytol. 1993;22:925–940. - PubMed
-
- Bronfman FC, Escudero CA, Weis J, Kruttgen A. Endosomal transport of neurotrophins: roles in signaling and neurodegenerative diseases. Dev Neurobiol. 2007;67:1183–1203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

