A general and efficient catalyst system for a Wacker-type oxidation using TBHP as the terminal oxidant: application to classically challenging substrates
- PMID: 19364100
- PMCID: PMC2763354
- DOI: 10.1021/ja901212h
A general and efficient catalyst system for a Wacker-type oxidation using TBHP as the terminal oxidant: application to classically challenging substrates
Abstract
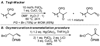
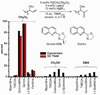
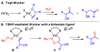
Utilizing the rapidly synthesized Quinox ligand and commercially available aqueous TBHP, a Wacker-type oxidation has been developed, which efficiently converts the traditionally challenging substrate class of protected allylic alcohols to the corresponding acyloin products. Additionally, the catalytic system is general for several other substrate classes, converting terminal olefins to methyl ketones, with short reaction times. The system is scalable (20 mmol) and can be performed with a reduced catalyst loading of 1 mol%. Enantioenriched substrates undergo oxidation with complete retention of enantiomeric excess.
Figures
References
-
-
For a recent review of Wacker oxidations, see: Takacs JM, Jiang X-t. Curr. Org. Chem. 2003;7:369–396.
- Tsuji J. Synthesis. 1984:369–384.
- Cornell CN, Sigman MS. Inorg. Chem. 2007;46:1903–1909. - PubMed
-
-
- Muzart J. Tetrahedron. 2007;63:7505–7521.
-
- Reetz MT. Angew. Chem., Int. Ed. Engl. 1984;23:556–569.
-
- Kang S-K, Jung K-Y, Chung J-U, Namkoong E-Y, Kim T-H. J. Org. Chem. 1995;60:4678–4679.
- Hamad O, Henry PM, Thompson C. J. Org. Chem. 1999;64:7745–7750.
-
-
For examples: Hunt DF, Rodeheaver GT. J. Chem. Soc. Chem. Comm. 1971:818–819.
- Crimmins MT, Brown BH. J. Am. Chem. Soc. 2004;126:10264–10266. - PubMed
- Nicolaou KC, Xu JY, Kim S, Pfefferkorn J, Ohshima T, Vourloumis D, Hosokawa S. J. Am. Chem. Soc. 1998;120:8661–8673.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

