Collisions or electrons? Protein sequence analysis in the 21st century
- PMID: 19364119
- PMCID: PMC2714553
- DOI: 10.1021/ac802330b
Collisions or electrons? Protein sequence analysis in the 21st century
Abstract
How dissociation is effected determines the upstream sample handling whereas the spectral features it produces regulate the downstream informatics approach. (To listen to a podcast about this feature, please go to the Analytical Chemistry website at pubs.acs.org/journal/ancham.
Figures
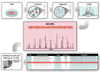





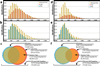


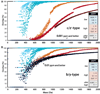

References
-
- McLafferty FW, Venktaraghavan R, Irving P. Biochemical and Biophysical Research Communications. 1970;39 - PubMed
-
- Wipf HK, Irving P, McCamish M, Venkataraghavan R, McLafferty FW. Journal of the American Chemical Society. 1973;95 - PubMed
-
- Zubarev RA, Kelleher NL, McLafferty FW. Journal of the American Chemical Society. 1998;120:3265–3266.
-
- Kruger NA, Zubarev RA, Horn DM, McLafferty FW. International Journal of Mass Spectrometry. 1999;187:787–793.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

