Susceptibility-weighted imaging: clinical angiographic applications
- PMID: 19364599
- PMCID: PMC2713115
- DOI: 10.1016/j.mric.2008.12.002
Susceptibility-weighted imaging: clinical angiographic applications
Abstract
By combining filtered phase and magnitude information to create a novel and intrinsic source of contrast, susceptibility-weighted imaging (SWI) has shown great promise in clinical angiography and venography. SWI has contributed to new insights into traumatic brain injury, the role of calcification in atherosclerosis, and the possible relationship between blood settling and deep venous thrombosis. A further contribution from SWI to deep venous thrombosis research (and also stroke) involves its application to the noninvasive measurement of oxygen saturation in the brain and in other tissues. Altogether, SWI offers manifold and diverse avenues for further research using angiographic and venographic techniques.
Figures
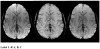

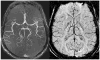

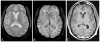






References
-
- Reichenbach JR, Venkatesan R, Schillinger DJ, et al. Small vessels in the human brain: MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology. 1997 Jul;204(1):272–277. - PubMed
-
- Haacke EM, Dmitriy SL, Yablonskiy A, et al. In vivo validation of the bold mechanism: A review of signal changes in gradient echo functional MRI in the presence of flow. International Journal of Imaging Systems and Technology. 1995;6(2–3):153–163.
-
- Ashwal S, Babikian T, Gardner-Nichols J, et al. Susceptibility-weighted imaging and proton magnetic resonance spectroscopy in assessment of outcome after pediatric traumatic brain injury. Arch Phys Med Rehabil. 2006 Dec;87(12 Suppl 2):S50–58. - PubMed
-
- Babikian T, Freier MC, Tong KA, et al. Susceptibility weighted imaging: neuropsychologic outcome and pediatric head injury. Pediatr Neurol. 2005 Sep;33(3):184–194. - PubMed
-
- Barth M, Nobauer-Huhmann IM, Reichenbach JR, et al. High-resolution three-dimensional contrast-enhanced blood oxygenation level-dependent magnetic resonance venography of brain tumors at 3 Tesla: first clinical experience and comparison with 1.5 Tesla. Invest Radiol. 2003 Jul;38(7):409–414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

