Responder analysis for pain relief and numbers needed to treat in a meta-analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice
- PMID: 19364730
- PMCID: PMC2800200
- DOI: 10.1136/ard.2009.107805
Responder analysis for pain relief and numbers needed to treat in a meta-analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice
Abstract
Background: Population mean changes from clinical trials are difficult to apply to individuals in clinical practice. Responder analysis may be better, but needs validating for level of response and treatment duration.
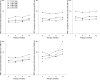
Methods: The numbers of patients with pain relief over baseline (> or =15%, > or =30%, > or =50%, > or =70%) at 2, 4, 8 and 12 weeks of treatment were obtained using the WOMAC 100 mm visual analogue pain subscale score for each treatment group in seven randomised placebo-controlled trials of etoricoxib in osteoarthritis lasting > or =6 weeks. Dropouts were assigned 0% improvement from baseline from then on. The numbers needed to treat (NNTs) were calculated at each level of response and time point.
Results: 3554 patients were treated with placebo, etoricoxib 30 mg and 60 mg, celecoxib 200 mg, naproxen 1000 mg or ibuprofen 2400 mg daily. Response rates fell with increasing pain relief: 60-80% experienced minimally important pain relief (> or =15%), 50-60% moderate pain relief (> or =30%), 40-50% substantial pain relief (> or =50%) and 20-30% extensive pain relief (> or =70%). NNTs for etoricoxib, celecoxib and naproxen were stable over 2-12 weeks. Ibuprofen showed lessening of effectiveness with time.
Conclusion: Responder rates and NNTs are reproducible for different levels of response over 12 weeks and have relevance for clinical practice at the individual patient level. An average 10 mm improvement in pain equates to almost one in two patients having substantial benefit.
Conflict of interest statement
RAM has received research grants, consulting or lecture fees from pharmaceutical companies including Pfizer, MSD, GSK, AstraZeneca, Grunenthal, Menarini, Futura and others. RAM and SD have also received research support from charities and government sources at various times. RAM is the guarantor. RAM, OAM and SD have no direct stock holding in any pharmaceutical company. PMP, ARG and HW are employees of Merck Inc.
Figures


References
-
- Christakis NA. Does this work for you? BMJ 2008;337:a2281
-
- Moore RA, Straube S, Derry S, et al. Does this work for you? Individuals, averages, and evidence based medicine. BMJ 2008;337:a2585. - PubMed
-
- Moore RA, Edwards JE, McQuay HJ. Acute pain: individual patient meta-analysis shows the impact of different ways of analysing and presenting results. Pain 2005;116:322–31 - PubMed
-
- Finnerup NB, Otto M, McQuay HJ, et al. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain 2005;118:289–305 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

