The role of tegumental aquaporin from the human parasitic worm, Schistosoma mansoni, in osmoregulation and drug uptake
- PMID: 19364765
- PMCID: PMC2717781
- DOI: 10.1096/fj.09-130757
The role of tegumental aquaporin from the human parasitic worm, Schistosoma mansoni, in osmoregulation and drug uptake
Abstract
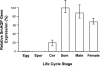
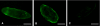
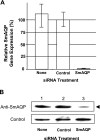
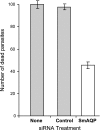
Schistosomes are parasitic platyhelminths that constitute an important public health problem globally. Infection is characterized by the presence of adult worms within the vasculature of their hosts, where they can reside for many years. The worms are covered by an unusual dual lipid bilayer through which they import nutrients. How the parasites import other vital molecules, such as water, is not known. Recent proteomic analysis of the schistosome tegumental membranes revealed the presence of an aquaporin homologue at the host-interactive surface whose cDNA we have cloned and characterized. The cDNA encodes a predicted 304-aa protein (SmAQP) that is found largely in the parasite tegument by immunolocalization and is most highly expressed in the intravascular life stages. Treatment of parasites with short interfering RNAs targeting the SmAQP gene results in potent (>90%) suppression. These suppressed parasites resist swelling when placed in hypotonic medium, unlike their control counterparts, which rapidly double in volume. In addition, SmAQP-suppressed parasites, unlike controls, resist shrinkage when incubated in hyperosmotic solution. While suppressed parasites exhibit lower viability in culture relative to controls and exhibit a stunted appearance following prolonged suppression, they are nonetheless more resistant to killing by the drug potassium antimonyl tartrate (PAT). This is likely because SmAQP acts as a conduit for this drug, as is the case for aquaporins in other systems. These experiments reveal a heretofore unrecognized role of the schistosome tegument in controlling water and drug movement into the parasites and highlight the importance of the tegument in parasite osmoregulation and drug uptake.
Figures

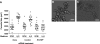
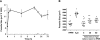
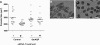
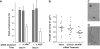
Similar articles
-
The tegument of the human parasitic worm Schistosoma mansoni as an excretory organ: the surface aquaporin SmAQP is a lactate transporter.PLoS One. 2010 May 3;5(5):e10451. doi: 10.1371/journal.pone.0010451. PLoS One. 2010. PMID: 20454673 Free PMC article.
-
Cloning the genes and DNA binding properties of High Mobility Group B1 (HMGB1) proteins from the human blood flukes Schistosoma mansoni and Schistosoma japonicum.Gene. 2006 Aug 1;377:33-45. doi: 10.1016/j.gene.2006.03.001. Epub 2006 Apr 27. Gene. 2006. PMID: 16644144
-
Amino acid transport in schistosomes: Characterization of the permeaseheavy chain SPRM1hc.J Biol Chem. 2007 Jul 27;282(30):21767-75. doi: 10.1074/jbc.M703512200. Epub 2007 Jun 1. J Biol Chem. 2007. PMID: 17545149
-
Metabolite movement across the schistosome surface.J Helminthol. 2012 Jun;86(2):141-7. doi: 10.1017/S0022149X12000120. Epub 2012 Feb 27. J Helminthol. 2012. PMID: 22365312 Review.
-
Tissue-specific transcriptome analyses provide new insights into GPCR signalling in adult Schistosoma mansoni.PLoS Pathog. 2018 Jan 18;14(1):e1006718. doi: 10.1371/journal.ppat.1006718. eCollection 2018 Jan. PLoS Pathog. 2018. PMID: 29346437 Free PMC article. Review.
Cited by
-
Nanomaterials as a Potential Target for Infectious Parasitic Agents.Curr Drug Deliv. 2024;21(6):828-851. doi: 10.2174/1567201820666230223085403. Curr Drug Deliv. 2024. PMID: 36815647 Review.
-
A Review of Nanotechnology for Targeted Anti-schistosomal Therapy.Front Bioeng Biotechnol. 2020 Jan 31;8:32. doi: 10.3389/fbioe.2020.00032. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32083071 Free PMC article. Review.
-
An atlas for Schistosoma mansoni organs and life-cycle stages using cell type-specific markers and confocal microscopy.PLoS Negl Trop Dis. 2011 Mar 8;5(3):e1009. doi: 10.1371/journal.pntd.0001009. PLoS Negl Trop Dis. 2011. PMID: 21408085 Free PMC article.
-
The human blood parasite Schistosoma mansoni expresses extracellular tegumental calpains that cleave the blood clotting protein fibronectin.Sci Rep. 2017 Oct 10;7(1):12912. doi: 10.1038/s41598-017-13141-5. Sci Rep. 2017. PMID: 29018227 Free PMC article.
-
Schistosome I/Lamides--a new family of bioactive helminth neuropeptides.Int J Parasitol. 2011 Jul;41(8):905-13. doi: 10.1016/j.ijpara.2011.03.010. Epub 2011 Apr 21. Int J Parasitol. 2011. PMID: 21554884 Free PMC article.
References
-
- Koukounari A, Gabrielli A F, Toure S, Bosque-Oliva E, Zhang Y, Sellin B, Donnelly C A, Fenwick A, Webster J P. Schistosoma haematobium infection and morbidity before and after large-scale administration of praziquantel in Burkina Faso. J Infect Dis. 2007;196:659–669. - PubMed
-
- King C H, Dickman K, Tisch D J. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. - PubMed
-
- King C H, Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chronic Illn. 2008;4:65–79. - PubMed
-
- Morris G P, Threadgold L T. Ultrastructure of the tegument of adult Schistosoma mansoni. J Parasitol. 1968;54:15–27. - PubMed
-
- Smith J H, Reynolds E S, Von Lichtenberg F. The integument of Schistosoma mansoni. Am J Trop Med Hyg. 1969;18:28–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

