Potent activity of indolequinones against human pancreatic cancer: identification of thioredoxin reductase as a potential target
- PMID: 19364812
- PMCID: PMC2701460
- DOI: 10.1124/mol.109.055855
Potent activity of indolequinones against human pancreatic cancer: identification of thioredoxin reductase as a potential target
Abstract
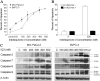
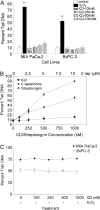
The indolequinone ES936 {5-methoxy-1,2-dimethyl-3-[(4-nitrophenoxy)methyl]indole-4,7-dione} was previously developed in our lab as an antitumor agent against pancreatic cancer. The objective of this study was to identify indolequinones with improved potency against pancreatic cancer and to define their mechanisms of action. Pancreatic cancer cell lines PANC-1, MIA PaCa-2, and BxPC-3 were used in in vitro assays [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) and clonogenic assays]; indolequinones displayed potent cytotoxicity against all three cell lines, and two specific classes of indolequinone were particularly potent agents. These indolequinones induced caspase-dependent apoptosis but no redox cycling or oxidative stress in MIA PaCa-2 and BxPC-3 cells. Selected indolequinones were also screened against the NCI-60 cell line panel and were found to be particularly effective against colon, renal, and melanoma cancer cells. A potential target of these indolequinones was identified as thioredoxin reductase. Indolequinones were found to be potent inhibitors of thioredoxin reductase activity both in pancreatic cancer cells and in cell-free systems. The mechanism of action of the indolequinones was shown to involve metabolic reduction, loss of a leaving group to generate a reactive electrophile resulting in alkylation of the selenocysteine residue in the active site of thioredoxin reductase. In vivo efficacy of the indolequinones was also tested in the MIA PaCa-2 pancreatic tumor xenograft in nude mice, and lead indolequinones demonstrated high efficacy and low toxicity. Inhibition of thioredoxin reductase represents a potential novel target in pancreatic cancer and may provide a biomarker of effect of lead indolequinones in this type of cancer.
Figures




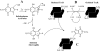
Similar articles
-
5-Methoxy-1,2-dimethyl-3-[(4-nitrophenoxy)methyl]indole-4,7-dione, a mechanism-based inhibitor of NAD(P)H:quinone oxidoreductase 1, exhibits activity against human pancreatic cancer in vitro and in vivo.Mol Cancer Ther. 2006 Jul;5(7):1702-9. doi: 10.1158/1535-7163.MCT-06-0105. Mol Cancer Ther. 2006. PMID: 16891456
-
Antitumor indolequinones induced apoptosis in human pancreatic cancer cells via inhibition of thioredoxin reductase and activation of redox signaling.Mol Pharmacol. 2012 Mar;81(3):401-10. doi: 10.1124/mol.111.076091. Epub 2011 Dec 6. Mol Pharmacol. 2012. PMID: 22147753 Free PMC article.
-
Development of indolequinone mechanism-based inhibitors of NAD(P)H:quinone oxidoreductase 1 (NQO1): NQO1 inhibition and growth inhibitory activity in human pancreatic MIA PaCa-2 cancer cells.Biochemistry. 2007 May 22;46(20):5941-50. doi: 10.1021/bi700008y. Epub 2007 Apr 25. Biochemistry. 2007. PMID: 17455910
-
Recent advances in the development of thioredoxin reductase inhibitors as anticancer agents.Curr Drug Targets. 2012 Oct;13(11):1432-44. doi: 10.2174/138945012803530224. Curr Drug Targets. 2012. PMID: 22876886 Review.
-
Thioredoxin reductase as a novel molecular target for cancer therapy.Cancer Lett. 2006 May 18;236(2):164-74. doi: 10.1016/j.canlet.2005.04.028. Epub 2005 Jun 13. Cancer Lett. 2006. PMID: 15955621 Review.
Cited by
-
A survey on the computational approaches to identify drug targets in the postgenomic era.Biomed Res Int. 2015;2015:239654. doi: 10.1155/2015/239654. Epub 2015 Apr 28. Biomed Res Int. 2015. PMID: 26060814 Free PMC article. Review.
-
Thioredoxin System Protein Expression in Carcinomas of the Pancreas, Distal Bile Duct, and Ampulla in the United Kingdom.Diseases. 2024 Sep 24;12(10):227. doi: 10.3390/diseases12100227. Diseases. 2024. PMID: 39452470 Free PMC article.
-
Interplay between autophagy and apoptosis in pancreatic tumors in response to gemcitabine.Target Oncol. 2014 Jun;9(2):123-34. doi: 10.1007/s11523-013-0278-5. Epub 2013 Apr 16. Target Oncol. 2014. PMID: 23588416
-
Cytotoxic and radiosensitising effects of a novel thioredoxin reductase inhibitor in breast cancer.Invest New Drugs. 2021 Oct;39(5):1232-1241. doi: 10.1007/s10637-021-01106-5. Epub 2021 Mar 25. Invest New Drugs. 2021. PMID: 33768386 Free PMC article.
-
Cytotoxic and Radiosensitising Effects of a Novel Thioredoxin Reductase Inhibitor in Brain Cancers.Mol Neurobiol. 2022 Jun;59(6):3546-3563. doi: 10.1007/s12035-022-02808-4. Epub 2022 Mar 28. Mol Neurobiol. 2022. PMID: 35344158 Free PMC article.
References
-
- Anestål K and Arnér ES (2003) Rapid induction of cell death by selenium-compromised thioredoxin reductase 1 but not by the fully active enzyme containing selenocysteine. J Biol Chem 278 15966-15972. - PubMed
-
- Arnér ES and Holmgren A (2006) The thioredoxin system in cancer. Semin Cancer Biol 16 420-426. - PubMed
-
- Beall HD, Winski S, Swann E, Hudnott AR, Cotterill AS, O'Sullivan N, Green SJ, Bien R, Siegel D, Ross D, et al. (1998) Indolequinone antitumor agents: correlation between quinone structure, rate of metabolism by recombinant human NAD(P)H: quinone oxidoreductase, and in vitro cytotoxicity. J Med Chem 41 4755-4766. - PubMed
-
- Bradshaw TD, Matthews CS, Cookson J, Chew EH, Shah M, Bailey K, Monks A, Harris E, Westwell AD, Wells G, et al. (2005) Elucidation of thioredoxin as a molecular target for antitumor quinols. Cancer Res 65 3911-3919. - PubMed
-
- Cassidy PB, Edes K, Nelson CC, Parsawar K, Fitzpatrick FA, and Moos PJ (2006) Thioredoxin reductase is required for the inactivation of tumor suppressor p53 and for apoptosis induced by endogenous electrophiles. Carcinogenesis 27 2538-2549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

