In vivo regulation of interleukin 1beta in patients with cryopyrin-associated periodic syndromes
- PMID: 19364880
- PMCID: PMC2715040
- DOI: 10.1084/jem.20082481
In vivo regulation of interleukin 1beta in patients with cryopyrin-associated periodic syndromes
Abstract
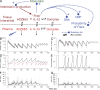
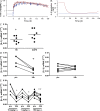
The investigation of interleukin 1beta (IL-1beta) in human inflammatory diseases is hampered by the fact that it is virtually undetectable in human plasma. We demonstrate that by administering the anti-human IL-1beta antibody canakinumab (ACZ885) to humans, the resulting formation of IL-1beta-antibody complexes allowed the detection of in vivo-produced IL-1beta. A two-compartment mathematical model was generated that predicted a constitutive production rate of 6 ng/d IL-1beta in healthy subjects. In contrast, patients with cryopyrin-associated periodic syndromes (CAPS), a rare monogenetic disease driven by uncontrolled caspase-1 activity and IL-1 production, produced a mean of 31 ng/d. Treatment with canakinumab not only induced long-lasting complete clinical response but also reduced the production rate of IL-1beta to normal levels within 8 wk of treatment, suggesting that IL-1beta production in these patients was mainly IL-1beta driven. The model further indicated that IL-1beta is the only cytokine driving disease severity and duration of response to canakinumab. A correction for natural IL-1 antagonists was not required to fit the data. Together, the study allowed new insights into the production and regulation of IL-1beta in man. It also indicated that CAPS is entirely mediated by IL-1beta and that canakinumab treatment restores physiological IL-1beta production.
Figures


References
-
- Dinarello C.A. 2002. The IL-1 family and inflammatory diseases.Clin. Exp. Rheumatol. 20:S1–S13 - PubMed
-
- Veltri S., Smith J.W. 1996. Interleukin 1 trials in cancer patients: a review of the toxicity, antitumor and hematopoietic effects.Stem Cells. 14:164–176 - PubMed
-
- Agostini L., Martinon F., Burns K., McDermott M.F., Hawkins P.N., Tschopp J. 2004. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder.Immunity. 20:319–325 - PubMed
-
- Dinarello C.A. 2004. Unraveling the NALP-3/IL-1beta inflammasome: a big lesson from a small mutation.Immunity. 20:243–244 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

