FAK alters invadopodia and focal adhesion composition and dynamics to regulate breast cancer invasion
- PMID: 19364917
- PMCID: PMC2700377
- DOI: 10.1083/jcb.200809110
FAK alters invadopodia and focal adhesion composition and dynamics to regulate breast cancer invasion
Abstract
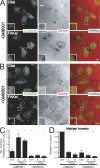
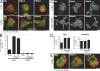
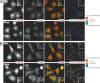
Focal adhesion kinase (FAK) is important for breast cancer progression and invasion and is necessary for the dynamic turnover of focal adhesions. However, it has not been determined whether FAK also regulates the dynamics of invasive adhesions formed in cancer cells known as invadopodia. In this study, we report that endogenous FAK functions upstream of cellular Src (c-Src) as a negative regulator of invadopodia formation and dynamics in breast cancer cells. We show that depletion of FAK induces the formation of active invadopodia but impairs invasive cell migration. FAK-deficient MTLn3 breast cancer cells display enhanced assembly and dynamics of invadopodia that are rescued by expression of wild-type FAK but not by FAK that cannot be phosphorylated at tyrosine 397. Moreover, our findings demonstrate that FAK depletion switches phosphotyrosine-containing proteins from focal adhesions to invadopodia through the temporal and spatial regulation of c-Src activity. Collectively, our findings provide novel insight into the interplay between FAK and Src to promote invasion.
Figures






References
-
- Artym V.V., Zhang Y., Seillier-Moiseiwitsch F., Yamada K.M., Mueller S.C. 2006. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function.Cancer Res. 66:3034–3043 - PubMed
-
- Bellis S.L., Miller J.T., Turner C.E. 1995. Characterization of tyrosine phosphorylation of paxillin in vitro by focal adhesion kinase.J. Biol. Chem. 270:17437–17441 - PubMed
-
- Bowden E.T., Onikoyi E., Slack R., Myoui A., Yoneda T., Yamada K.M., Mueller S.C. 2006. Co-localization of cortactin and phosphotyrosine identifies active invadopodia in human breast cancer cells.Exp. Cell Res. 312:1240–1253 - PubMed
-
- Brabek J., Constancio S.S., Siesser P.F., Shin N.Y., Pozzi A., Hanks S.K. 2005. Crk-associated substrate tyrosine phosphorylation sites are critical for invasion and metastasis of SRC-transformed cells.Mol. Cancer Res. 3:307–315 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

