Regulatory T cells are reduced during anti-CD25 antibody treatment of multiple sclerosis
- PMID: 19364932
- PMCID: PMC2669544
- DOI: 10.1001/archneurol.2009.16
Regulatory T cells are reduced during anti-CD25 antibody treatment of multiple sclerosis
Abstract
Objective: Maintenance therapy with anti-CD25 antibody has emerged as a potentially useful treatment for multiple sclerosis (MS). Constitutive CD25 expression on CD4+CD25+ regulatory T cells (Treg) suggests that anti-CD25 antibody treatment may potentially target a subset of T cells that exhibit immune suppressive properties. We examined changes to CD4+CD25+ Treg in patients with MS receiving maintenance anti-CD25 monoclonal antibody treatment to determine the effect of treatment on Treg and, consequently, on immunological tolerance.
Design: Peripheral blood and cerebrospinal fluid samples obtained from a before-and-after trial of anti-CD25 antibody monotherapy were examined to compare baseline and treatment differences in CD4+CD25+ Treg.
Subjects: A total of 15 subjects with MS. One subject was withdrawn owing to an adverse effect.
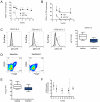
Results: Sustained reduction of the frequency of CD4+CD25+ Treg was observed during treatment. Anti-CD25 antibody treatment led to evidence of impaired in vivo Treg proliferation and impaired ex vivo Treg suppression. Inflammatory MS activity was substantially reduced with treatment despite reduction of circulating Treg, and there was no correlation between changes in the frequency of Treg and changes in brain inflammatory activity. However, new-onset inflammatory disease, notably dermatitis, was also observed in a number of subjects during treatment.
Conclusion: The reduction in Treg did not negatively affect maintenance of central nervous system tolerance during anti-CD25 antibody treatment. The incidence of new-onset inflammatory disease outside of the central nervous system in a subset of patients, however, warrants further studies to examine the possibility of compartmental differences in the capacity to maintain tolerance in the setting of reduced CD4+CD25+ Treg.
Figures




References
-
- Waldmann TA. The IL-2/IL-2 receptor system: a target for rational immune intervention. Immunol Today. 1993;14:264–270. - PubMed
-
- Rose JW, Burns JB, Bjorklund J, et al. Daclizumab phase II trial in relapsing and remitting multiple sclerosis: MRI and clinical results. Neurology. 2007;69:785–789. - PubMed
-
- Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

