Phase I study of samarium-153 lexidronam with docetaxel in castration-resistant metastatic prostate cancer
- PMID: 19364960
- PMCID: PMC2684850
- DOI: 10.1200/JCO.2008.20.4164
Phase I study of samarium-153 lexidronam with docetaxel in castration-resistant metastatic prostate cancer
Erratum in
- J Clin Oncol. 2011 Oct 10;29(29):3947-8
Abstract
Purpose: Early studies of patients with castration-resistant metastatic prostate cancer (CRMPC) suggest that chemotherapy administered with a dose of a bone-seeking radiopharmaceutical is superior to chemotherapy alone. To build on this strategy and fully integrate a repetitively dosed bone-seeking radiopharmaceutical into a contemporary chemotherapy regimen, we conducted a phase I study of docetaxel and samarium-153 ((153)Sm) lexidronam.
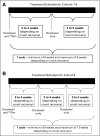
Patients and methods: Men with progressive CRMPC were eligible. Cohorts of three to six patients were defined by dose escalations as follows: docetaxel 65, 70, 75, 75, 75 mg/m(2) and (153)Sm ethylenediaminetetramethylenephosphonate (EDTMP) 0.5, 0.5, 0.5, 0.75, 1 mCi/kg. Each cycle lasted a minimum of 6 (cohorts 1 through 5) or 9 (cohort 6) weeks. Docetaxel was administered on days 1 and 22 (and day 43 for cohort 6), and (153)Sm-EDTMP was administered on day -1 to 1 of each cycle. Patients with acceptable hematologic toxicities were eligible to receive additional cycles until progression.
Results: Twenty-eight men were treated in six cohorts. Maximum-tolerated dose was not reached, because full doses of both agents were well tolerated, even using an every-6-week dosing schedule of (153)Sm-EDTMP. Patients received an average of 5.6 docetaxel doses (range, one to 13 doses) and 2.9 (153)Sm-EDTMP doses (range, one to six doses). Fifteen patients demonstrated a more than 50% decline in prostate-specific antigen. Treatment significantly reduced indices of bone deposition and resorption.
Conclusion: Docetaxel and (153)Sm-EDTMP can be combined safely at full doses over repeated cycles. Responses were seen in the small group of patients with taxane-resistant disease tested. The optimal phase II doses for patients with taxane-naïve disease may differ from those optimal for patients with taxane-resistant disease.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


Comment in
-
Radiopharmaceutical and chemotherapy combinations in metastatic castrate-resistant prostate cancer: a new beginning?J Clin Oncol. 2009 May 20;27(15):2417-8. doi: 10.1200/JCO.2008.21.4460. Epub 2009 Apr 13. J Clin Oncol. 2009. PMID: 19364953 No abstract available.
References
-
- Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. - PubMed
-
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. - PubMed
-
- Tu SM, Millikan RE, Mengistu B, et al. Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: A randomised phase II trial. Lancet. 2001;357:336–341. - PubMed
-
- Higano CS, Quick DP, Bushnell D, et al. Safety analysis of repeated high doses of samarium-153 lexidronam in men with hormone-naive prostate cancer metastatic to bone. Clin Genitourin Cancer. 2008;6:40–45. - PubMed
-
- Sartor O, Reid RH, Bushnell DL, et al. Safety and efficacy of repeat administration of samarium Sm-153 lexidronam to patients with metastatic bone pain. Cancer. 2007;109:637–643. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

