Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma
- PMID: 19364962
- PMCID: PMC3646305
- DOI: 10.1200/JCO.2008.20.3943
Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma
Abstract
Purpose: Neoadjuvant pemetrexed plus cisplatin was administered, followed by extrapleural pneumonectomy (EPP) and hemithoracic radiation (RT), to assess the feasibility and efficacy of trimodality therapy in stage I to III malignant pleural mesothelioma.
Patients and methods: Requirements included stage T1-3 N0-2 disease, no prior surgical resection, adequate organ function (including predicted postoperative forced expiratory volume in 1 second > or = 35%), and performance status 0 to 1. Patients received pemetrexed 500 mg/m(2) plus cisplatin 75 mg/m(2) for four cycles. Patients without disease progression underwent EPP followed by RT (54 Gy). The primary end point was pathologic complete response (pCR) rate.
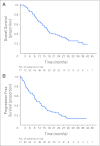
Results: Seventy-seven patients received chemotherapy. All four cycles were administered to 83% of patients. The radiologic response rate was 32.5% (95% CI, 22.2 to 44.1). Fifty-seven patients proceeded to EPP, which was completed in 54 patients. Three pCRs were observed (5% of EPP). Forty of 44 patients completed irradiation. Median survival in the overall population was 16.8 months (95% CI, 13.6 to 23.2 months; censorship, 33.8%). Patients completing all therapy had a median survival of 29.1 months and a 2-year survival rate of 61.2%. Radiologic response of complete or partial response was associated with a median survival of 26.0 months compared with 13.9 months for patients with stable disease or progressive disease (P = .05).
Conclusion: This multicenter trial showed that trimodality therapy with neoadjuvant pemetrexed plus cisplatin is feasible with a reasonable long-term survival rate, particularly for patients who completed all therapy. Radiologic response to chemotherapy, but not sex, histology, disease stage, or nodal status, was associated with improved survival.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Price B. Analysis of current trends in United States mesothelioma incidence. Am J Epidemiol. 1997;145:211–218. - PubMed
-
- Zellos L, Christiani DC. Epidemiology, biologic behavior, and natural history of mesothelioma. Thorac Surg Clin. 2004;14:469–477. - PubMed
-
- van Ruth S, Baas P, Zoetmulder FA. Surgical treatment of malignant pleural mesothelioma: A review. Chest. 2003;123:551–561. - PubMed
-
- Baldini EH. External beam radiation therapy for the treatment of pleural mesothelioma. Thorac Surg Clin. 2004;14:543–548. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical