A right-sided pathway involving FGF8/Snai1 controls asymmetric development of the proepicardium in the chick embryo
- PMID: 19365073
- PMCID: PMC2678653
- DOI: 10.1073/pnas.0811944106
A right-sided pathway involving FGF8/Snai1 controls asymmetric development of the proepicardium in the chick embryo
Abstract
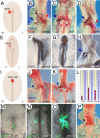
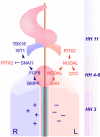
The proepicardium (PE) is a transient structure that forms at the venous pole of the embryonic vertebrate heart. This cardiac progenitor cell population gives rise to the epicardium, coronary vasculature, and fibroblasts. In the chicken embryo, the PE displays left-right (L-R) asymmetry and develops only on the right side, while on the left only a vestigial PE is formed, which subsequently gets lost by apoptosis. In this study, we analyzed how the L-R asymmetry pathway affects PE formation. Experimental manipulation of left-side determinants such as Shh, Nodal, and Cfc as well as forced expression of Pitx2 had no effect on the sidedness of PE development. In contrast, inhibition of early-acting regulators of L-R axis formation such as H(+)/K(+)-ATPase or primitive streak apoptosis affected the sidedness of PE development. Experimental interference with the right-side determinants Fgf8 or Snai1 prevented PE formation, whereas ectopic left-sided expression of Fgf8 or Snai1 resulted in bilateral PE development. These data provide novel insight into the molecular control of asymmetric morphogenesis suggesting that also the right side harbors an instructive signaling pathway that is involved in the control of PE development. This pathway might be of general relevance for setting up L-R asymmetries at the venous pole of the heart.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Raya A, Belmonte JC. Left-right asymmetry in the vertebrate embryo: From early information to higher-level integration. Nat Rev Genet. 2006;7:283–293. - PubMed
-
- Schlueter J, Brand T. Left-right axis development: Examples of similar and divergent strategies to generate asymmetric morphogenesis in chick and mouse embryos. Cytogenet Genome Res. 2007;117:256–267. - PubMed
-
- Lopez-Gracia ML, Ros MA. Left-right asymmetry in vertebrate development. Adv Anat Embryol Cell Biol. 2007;188:1–121. - PubMed
-
- Levin M, et al. Asymmetries in H+/K+-ATPase and cell membrane potentials comprise a very early step in left-right patterning. Cell. 2002;111:77–89. - PubMed
-
- Levin M, Palmer AR. Left-right patterning from the inside out: Widespread evidence for intracellular control. BioEssays. 2007;29:271–287. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

