Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity
- PMID: 19365083
- PMCID: PMC2699231
- DOI: 10.1182/blood-2008-06-165225
Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity
Abstract
Natural killer (NK) cells serve as important effectors for antitumor immunity, and CD56+CD45+ NK cells can be routinely derived from human embryonic stem cells (hESCs). However, little is know about the ability of hESC-derived NK cells to mediate an effective in vivo antitumor response. Using bioluminescent imaging, we now demonstrate that H9 line hESC-derived NK cells mediate effective clearance of human tumor cells in vivo. In addition to increased in vitro killing of diverse tumor targets, the in vivo tumor clearance by H9 hESC-derived NK cells was more effective compared with NK cells derived from umbilical cord blood (UCB). Phenotypic analysis demonstrates the hESC-derived NK cells are uniformly CD94+CD117(low/-), an NK-cell population characterized by potent cytolytic activity and thus more competent to mediate tumor clearance. These studies demonstrate that hESCs provide an important model to study human lymphocyte development and may serve as a novel source for antitumor immunotherapy.
Figures


 ]), and hESC-NK–treated (n = 13, ●) mice. Error bars indicated SEM. *P < .01 for hESC-NK vs UCB-NK and control. (C) CD56+ NK cells were magnetically sorted and injected intravenously into tumor-bearing mice. Mice treated with either sorted CD56+ hESC-NK cells (n = 3, —) or sorted CD56+ UCB-NK cells (n = 2,
]), and hESC-NK–treated (n = 13, ●) mice. Error bars indicated SEM. *P < .01 for hESC-NK vs UCB-NK and control. (C) CD56+ NK cells were magnetically sorted and injected intravenously into tumor-bearing mice. Mice treated with either sorted CD56+ hESC-NK cells (n = 3, —) or sorted CD56+ UCB-NK cells (n = 2,  ) demonstrate tumor regression similar to mice treated with unsorted hESC- or UCB-NK cells.
) demonstrate tumor regression similar to mice treated with unsorted hESC- or UCB-NK cells.
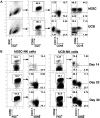

 ) at the indicated effector-to-target cell ratios. Representative results from 2 separate experiments are shown. (E) Flow cytometric analysis of cell trafficking molecules on hESC- and UCB-derived NK cells. Histograms of CD56+-gated NK cells are shown. Open histogram indicates isotype control.
) at the indicated effector-to-target cell ratios. Representative results from 2 separate experiments are shown. (E) Flow cytometric analysis of cell trafficking molecules on hESC- and UCB-derived NK cells. Histograms of CD56+-gated NK cells are shown. Open histogram indicates isotype control.References
-
- Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2:850–861. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

