Fluorescence and electron microscopic localization of F-actin in the ependymocytes
- PMID: 19365089
- PMCID: PMC2713074
- DOI: 10.1369/jhc.2009.953646
Fluorescence and electron microscopic localization of F-actin in the ependymocytes
Abstract
The organization of F-actin in the ventricular system has been reported to display pronounced regional differences with respect to shape, size, and development. However, the real roles played by F-actin in these cells cannot be understood unless the precise localization of F-actin is defined. In the present study, we used double-fluorescence labeling to further examine the localization of F-actin in the ependymocytes and its spatial relation to the other two cytoskeletal components, microtubules and intermediate filaments. Then we converted fluorescence signals for F-actin to peroxidase/DAB reaction products by use of a phalloidin-based FITC-anti-FITC system. This detection technique provided an overview of the distribution of F-actin in the ependymocytes at the ultrastructural level, and has been proven to be helpful in correlating light and electron microscopic investigations.
Figures
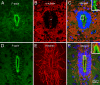
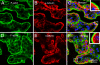





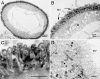
Similar articles
-
Regionally varying F-actin network in the apical cytoplasm of ependymocytes.Neurosci Res. 2007 Apr;57(4):522-30. doi: 10.1016/j.neures.2006.12.009. Epub 2006 Dec 27. Neurosci Res. 2007. PMID: 17239462
-
The unique organization of filamentous actin in the medullary canal of the medulla oblongata.Tissue Cell. 2017 Apr;49(2 Pt B):336-344. doi: 10.1016/j.tice.2017.01.005. Epub 2017 Jan 24. Tissue Cell. 2017. PMID: 28187870
-
Radial astrocytes and ependymocytes in the spinal cord of the adult toad (Bufo bufo L.). An immunohistochemical and ultrastructural study.Cell Tissue Res. 1990 May;260(2):307-14. doi: 10.1007/BF00318633. Cell Tissue Res. 1990. PMID: 2113429
-
A tridimensional view of the organization of actin filaments in the central nervous system by use of fluorescent photooxidation.Biocell. 2008 Apr;32(1):1-8. Biocell. 2008. PMID: 18669318 Review.
-
Ependymal and choroidal cells in culture: characterization and functional differentiation.Microsc Res Tech. 1998 Apr 15;41(2):124-57. doi: 10.1002/(SICI)1097-0029(19980415)41:2<124::AID-JEMT3>3.0.CO;2-U. Microsc Res Tech. 1998. PMID: 9579599 Review.
Cited by
-
Tachycardia and hypertension enhance tracer efflux from the spinal cord.Fluids Barriers CNS. 2021 Oct 26;18(1):47. doi: 10.1186/s12987-021-00279-8. Fluids Barriers CNS. 2021. PMID: 34702290 Free PMC article.
-
Stretching morphogenesis of the roof plate and formation of the central canal.PLoS One. 2013;8(2):e56219. doi: 10.1371/journal.pone.0056219. Epub 2013 Feb 7. PLoS One. 2013. PMID: 23409159 Free PMC article.
-
Histochemical localization of caldesmon in the CNS and ganglia of the mouse.J Histochem Cytochem. 2011 May;59(5):504-17. doi: 10.1369/0022155411400875. Epub 2011 Mar 16. J Histochem Cytochem. 2011. PMID: 21411712 Free PMC article.
-
Transcytosis in the blood-cerebrospinal fluid barrier of the mouse brain with an engineered receptor/ligand system.Mol Ther Methods Clin Dev. 2015 Oct 7;2:15037. doi: 10.1038/mtm.2015.37. eCollection 2015. Mol Ther Methods Clin Dev. 2015. PMID: 26491705 Free PMC article.
-
COVID-19 Pandemic and Its Impact on Neurosurgery Practice in Malaysia: Academic Insights, Clinical Experience and Protocols from March till August 2020.Malays J Med Sci. 2020 Oct;27(5):141-195. doi: 10.21315/mjms2020.27.5.14. Epub 2020 Oct 27. Malays J Med Sci. 2020. PMID: 33154710 Free PMC article.
References
-
- Bray D, White JG (1988) Cortical flow in animal cells. Science 239:883–888 - PubMed
-
- Capani F, Deerinck TJ, Ellisman MH, Bushong E, Bobik M, Martone ME (2001) Phalloidin-eosin followed by photo-oxidation: a novel method for localizing F-actin at the light and electron microscopic levels. J Histochem Cytochem 49:1351–1361 - PubMed
-
- Castellani-Ceresa L, Brivio MF, Radaelli G (1991) Electron microscopic localization of F-actin in acrosome reacted boar spermatozoa by means of a phalloidin-FITC complex. J Submicrosc Cytol Pathol 23:347–349 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

