Esterified cholesterol is highly localized to Bruch's membrane, as revealed by lipid histochemistry in wholemounts of human choroid
- PMID: 19365091
- PMCID: PMC2713073
- DOI: 10.1369/jhc.2009.953448
Esterified cholesterol is highly localized to Bruch's membrane, as revealed by lipid histochemistry in wholemounts of human choroid
Abstract
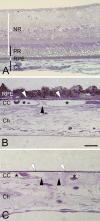
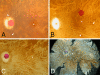
Accumulation of neutral lipids in Bruch's membrane (BrM) is a major age change in human retina and contributes to the formation of extracellular lesions associated with age-related macular degeneration. We developed a BrM-choroid wholemounting technique suitable for reliable staining and evaluated different fluorescent lipid dyes for topographic semiquantitative analysis of BrM lipids. Thin BrM-choroid complexes with partially stripped choroid from 10 aged donor eyes were prepared with an optimized wholemounting technique. Preparation quality was monitored by examining 1-mum-thick sections of representative samples. The staining patterns of Nile Red, BODIPY 493/503, filipin for unesterified cholesterol (UC-F), filipin for esterified cholesterol (EC-F), and Oil Red O in wholemounts were compared with their staining patterns in chorioretinal sections, using wide-field epi-fluorescence microscopy. Wholemounts exhibited optimal flatness on the BrM side. Reduced tissue thickness allowed reliable dye penetration and staining of BrM. Only EC-F was with high specificity localized to BrM and demonstrated an intense and distinct granular staining pattern not previously appreciated in chorioretinal sections. All other lipid dyes also stained choroidal or retinal tissue intensely. No dye provided perfect characteristics in regard to representing all neutral lipid classes present in BrM or to fluorescence intensity. Nevertheless, only EC-F was highly localized to BrM with a specific granular pattern. Because direct assays indicate that esterified cholesterol is abundantly present in BrM, we consider EC-F the most valuable choice for analyzing neutral lipid deposits in human BrM.
Figures


Similar articles
-
Detection of esterified cholesterol in murine Bruch's membrane wholemounts with a perfringolysin O-based cholesterol marker.Invest Ophthalmol Vis Sci. 2014 Jul 1;55(8):4759-67. doi: 10.1167/iovs.14-14311. Invest Ophthalmol Vis Sci. 2014. PMID: 24985479
-
Accumulation of cholesterol with age in human Bruch's membrane.Invest Ophthalmol Vis Sci. 2001 Jan;42(1):265-74. Invest Ophthalmol Vis Sci. 2001. PMID: 11133878
-
Internal structure consistent with remodelling in very small drusen, revealed by filipin histochemistry for esterified cholesterol.Br J Ophthalmol. 2014 May;98(5):698-702. doi: 10.1136/bjophthalmol-2013-304226. Epub 2014 Feb 19. Br J Ophthalmol. 2014. PMID: 24554738
-
[A new approach for studying the retinal and choroidal circulation].Nippon Ganka Gakkai Zasshi. 2004 Dec;108(12):836-61; discussion 862. Nippon Ganka Gakkai Zasshi. 2004. PMID: 15656089 Review. Japanese.
-
Apolipoprotein B-containing lipoproteins in retinal aging and age-related macular degeneration.J Lipid Res. 2010 Mar;51(3):451-67. doi: 10.1194/jlr.R002238. Epub 2009 Sep 29. J Lipid Res. 2010. PMID: 19797256 Free PMC article. Review.
Cited by
-
Multiplex analysis of age-related protein and lipid modifications in human Bruch's membrane.FASEB J. 2010 Dec;24(12):4816-24. doi: 10.1096/fj.10-166090. Epub 2010 Aug 4. FASEB J. 2010. PMID: 20686107 Free PMC article.
-
Soft Drusen in Age-Related Macular Degeneration: Biology and Targeting Via the Oil Spill Strategies.Invest Ophthalmol Vis Sci. 2018 Mar 20;59(4):AMD160-AMD181. doi: 10.1167/iovs.18-24882. Invest Ophthalmol Vis Sci. 2018. PMID: 30357336 Free PMC article. Review.
-
Cell-Matrix Interactions in the Eye: From Cornea to Choroid.Cells. 2021 Mar 20;10(3):687. doi: 10.3390/cells10030687. Cells. 2021. PMID: 33804633 Free PMC article. Review.
-
Retinal pigment epithelium-specific CLIC4 mutant is a mouse model of dry age-related macular degeneration.Nat Commun. 2022 Jan 18;13(1):374. doi: 10.1038/s41467-021-27935-9. Nat Commun. 2022. PMID: 35042858 Free PMC article.
-
Serum starvation of ARPE-19 changes the cellular distribution of cholesterol and Fibulin3 in patterns reminiscent of age-related macular degeneration.Exp Cell Res. 2017 Dec 15;361(2):333-341. doi: 10.1016/j.yexcr.2017.10.036. Epub 2017 Oct 31. Exp Cell Res. 2017. PMID: 29097185 Free PMC article.
References
-
- Adams CWM, Bayliss OB (1975) Lipid histochemistry. In Glick D, Rosenbaum RM, eds. Techniques of Biochemical and Biophysical Morphology. 2nd ed. New York, John Wiley and Sons, 100–156
-
- Beatty S, Koh H, Phil M, Henson D, Boulton M (2000) The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol 45:115–134 - PubMed
-
- Chong NH, Keonin J, Luthert PJ, Frennesson CI, Weingeist DM, Wolf RL, Mullins RF, et al. (2005) Decreased thickness and integrity of the macular elastic layer of Bruch's membrane correspond to the distribution of lesions associated with age-related macular degeneration. Am J Pathol 166:241–251 - PMC - PubMed
-
- Curcio CA, Millican CL, Bailey T, Kruth HS (2001) Accumulation of cholesterol with age in human Bruch's membrane. Invest Ophthalmol Vis Sci 42:265–274 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

