Beta-carbonic anhydrases play a role in fruiting body development and ascospore germination in the filamentous fungus Sordaria macrospora
- PMID: 19365544
- PMCID: PMC2664464
- DOI: 10.1371/journal.pone.0005177
Beta-carbonic anhydrases play a role in fruiting body development and ascospore germination in the filamentous fungus Sordaria macrospora
Abstract
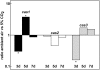
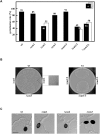
Carbon dioxide (CO(2)) is among the most important gases for all organisms. Its reversible interconversion to bicarbonate (HCO(3) (-)) reaches equilibrium spontaneously, but slowly, and can be accelerated by a ubiquitous group of enzymes called carbonic anhydrases (CAs). These enzymes are grouped by their distinct structural features into alpha-, beta-, gamma-, delta- and zeta-classes. While physiological functions of mammalian, prokaryotic, plant and algal CAs have been extensively studied over the past years, the role of beta-CAs in yeasts and the human pathogen Cryptococcus neoformans has been elucidated only recently, and the function of CAs in multicellular filamentous ascomycetes is mostly unknown. To assess the role of CAs in the development of filamentous ascomycetes, the function of three genes, cas1, cas2 and cas3 (carbonic anhydrase of Sordaria) encoding beta-class carbonic anhydrases was characterized in the filamentous ascomycetous fungus Sordaria macrospora. Fluorescence microscopy was used to determine the localization of GFP- and DsRED-tagged CAs. While CAS1 and CAS3 are cytoplasmic enzymes, CAS2 is localized to the mitochondria. To assess the function of the three isoenzymes, we generated knock-out strains for all three cas genes (Deltacas1, Deltacas2, and Deltacas3) as well as all combinations of double mutants. No effect on vegetative growth, fruiting-body and ascospore development was seen in the single mutant strains lacking cas1 or cas3, while single mutant Deltacas2 was affected in vegetative growth, fruiting-body development and ascospore germination, and the double mutant strain Deltacas1/2 was completely sterile. Defects caused by the lack of cas2 could be partially complemented by elevated CO(2) levels or overexpression of cas1, cas3, or a non-mitochondrial cas2 variant. The results suggest that CAs are required for sexual reproduction in filamentous ascomycetes and that the multiplicity of isoforms results in redundancy of specific and non-specific functions.
Conflict of interest statement
Figures




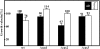


Similar articles
-
The filamentous ascomycete Sordaria macrospora can survive in ambient air without carbonic anhydrases.Mol Microbiol. 2014 Jun;92(5):931-44. doi: 10.1111/mmi.12607. Epub 2014 May 2. Mol Microbiol. 2014. PMID: 24720701
-
Autophagy genes Smatg8 and Smatg4 are required for fruiting-body development, vegetative growth and ascospore germination in the filamentous ascomycete Sordaria macrospora.Autophagy. 2013 Jan;9(1):33-49. doi: 10.4161/auto.22398. Epub 2012 Oct 12. Autophagy. 2013. PMID: 23064313 Free PMC article.
-
Crystal structures of two tetrameric β-carbonic anhydrases from the filamentous ascomycete Sordaria macrospora.FEBS J. 2014 Apr;281(7):1759-72. doi: 10.1111/febs.12738. Epub 2014 Feb 19. FEBS J. 2014. PMID: 24506675
-
Carbonic anhydrases in fungi.Microbiology (Reading). 2010 Jan;156(Pt 1):23-29. doi: 10.1099/mic.0.032581-0. Epub 2009 Oct 15. Microbiology (Reading). 2010. PMID: 19833770 Review.
-
A matter of structure: structural comparison of fungal carbonic anhydrases.Appl Microbiol Biotechnol. 2014 Oct;98(20):8433-41. doi: 10.1007/s00253-014-5993-z. Epub 2014 Aug 12. Appl Microbiol Biotechnol. 2014. PMID: 25109265 Review.
Cited by
-
Lessons on fruiting body morphogenesis from genomes and transcriptomes of Agaricomycetes.Stud Mycol. 2023 Jul;104:1-85. doi: 10.3114/sim.2022.104.01. Epub 2023 Jan 31. Stud Mycol. 2023. PMID: 37351542 Free PMC article.
-
Autophagy-Associated Protein SmATG12 Is Required for Fruiting-Body Formation in the Filamentous Ascomycete Sordaria macrospora.PLoS One. 2016 Jun 16;11(6):e0157960. doi: 10.1371/journal.pone.0157960. eCollection 2016. PLoS One. 2016. PMID: 27309377 Free PMC article.
-
β carbonic anhydrase is required for female fertility in Drosophila melanogaster.Front Zool. 2015 Aug 22;12:19. doi: 10.1186/s12983-015-0111-3. eCollection 2015. Front Zool. 2015. PMID: 26300950 Free PMC article.
-
Molecular and biochemical characterization of carbonic anhydrases of Paracoccidioides.Genet Mol Biol. 2016 Jul-Sep;39(3):416-25. doi: 10.1590/1678-4685-GMB-2015-0213. Epub 2016 Jul 25. Genet Mol Biol. 2016. PMID: 27560991 Free PMC article.
-
Molecular and biochemical analysis of the beta class carbonic anhydrases in Caenorhabditis elegans.Mol Biol Rep. 2010 Jul;37(6):2941-50. doi: 10.1007/s11033-009-9857-z. Epub 2009 Oct 9. Mol Biol Rep. 2010. PMID: 19816790
References
-
- Casey JR. Why bicarbonate? Biochem Cell Biol. 2006;84:930–939. - PubMed
-
- Supuran CT. Carbonic anhydrases–an overview. Curr Pharm Des. 2008;14:603–614. - PubMed
-
- Tripp BC, Smith K, Ferry JG. Carbonic anhydrase: new insights for an ancient enzyme. J Biol Chem. 2001;276:48615–48618. - PubMed
-
- Hewett-Emmett D, Tashian RE. Functional diversity, conservation, and convergence in the evolution of the alpha-, beta-, and gamma-carbonic anhydrase gene families. Mol Phylogenet Evol. 1996;5:50–77. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

