Smoothened adopts multiple active and inactive conformations capable of trafficking to the primary cilium
- PMID: 19365551
- PMCID: PMC2664476
- DOI: 10.1371/journal.pone.0005182
Smoothened adopts multiple active and inactive conformations capable of trafficking to the primary cilium
Abstract
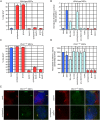
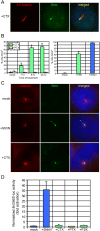
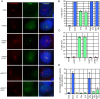
Activation of Hedgehog (Hh) signaling requires the transmembrane protein Smoothened (Smo), a member of the G-protein coupled receptor superfamily. In mammals, Smo translocates to the primary cilium upon binding of Hh ligands to their receptor, Patched (Ptch1), but it is unclear if ciliary trafficking of Smo is sufficient for pathway activation. Here, we demonstrate that cyclopamine and jervine, two structurally related inhibitors of Smo, force ciliary translocation of Smo. Treatment with SANT-1, an unrelated Smo antagonist, abrogates cyclopamine- and jervine-mediated Smo translocation. Further, activation of protein kinase A, either directly or through activation of Galphas, causes Smo to translocate to a proximal region of the primary cilium. We propose that Smo adopts multiple inactive and active conformations, which influence its localization and trafficking on the primary cilium.
Conflict of interest statement
Figures

References
-
- McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. 2003;53:1–114. - PubMed
-
- Taipale J, Cooper MK, Maiti T, Beachy PA. Patched acts catalytically to suppress the activity of Smoothened. Nature. 2002;418:892–897. - PubMed
-
- Huangfu D, Anderson KV. Signaling from Smo to Ci/Gli: conservation and divergence of Hedgehog pathways from Drosophila to vertebrates. Development. 2006;133:3–14. - PubMed
-
- Varjosalo M, Li SP, Taipale J. Divergence of hedgehog signal transduction mechanism between Drosophila and mammals. Dev Cell. 2006;10:177–186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

