Low enzymatic activity haplotypes of the human catechol-O-methyltransferase gene: enrichment for marker SNPs
- PMID: 19365560
- PMCID: PMC2664927
- DOI: 10.1371/journal.pone.0005237
Low enzymatic activity haplotypes of the human catechol-O-methyltransferase gene: enrichment for marker SNPs
Abstract
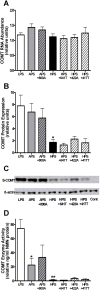
Catechol-O-methyltransferase (COMT) is an enzyme that plays a key role in the modulation of catechol-dependent functions such as cognition, cardiovascular function, and pain processing. Three common haplotypes of the human COMT gene, divergent in two synonymous and one nonsynonymous (val(158)met) position, designated as low (LPS), average (APS), and high pain sensitive (HPS), are associated with experimental pain sensitivity and risk of developing chronic musculoskeletal pain conditions. APS and HPS haplotypes produce significant functional effects, coding for 3- and 20-fold reductions in COMT enzymatic activity, respectively. In the present study, we investigated whether additional minor single nucleotide polymorphisms (SNPs), accruing in 1 to 5% of the population, situated in the COMT transcript region contribute to haplotype-dependent enzymatic activity. Computer analysis of COMT ESTs showed that one synonymous minor SNP (rs769224) is linked to the APS haplotype and three minor SNPs (two synonymous: rs6267, rs740602 and one nonsynonymous: rs8192488) are linked to the HPS haplotype. Results from in silico and in vitro experiments revealed that inclusion of allelic variants of these minor SNPs in APS or HPS haplotypes did not modify COMT function at the level of mRNA folding, RNA transcription, protein translation, or enzymatic activity. These data suggest that neutral variants are carried with APS and HPS haplotypes, while the high activity LPS haplotype displays less linked variation. Thus, both minor synonymous and nonsynonymous SNPs in the coding region are markers of functional APS and HPS haplotypes rather than independent contributors to COMT activity.
Conflict of interest statement
Figures
References
-
- Lundstrom K, Salminen M, Jalanko A, Savolainen R, Ulmanen I. Cloning and characterization of human placental catechol-O-methyltransferase cDNA. DNA Cell Biol. 1991;10:181–189. - PubMed
-
- Tenhunen J, Salminen M, Jalanko A, Ukkonen S, Ulmanen I. Structure of the rat catechol-O-methyltransferase gene: separate promoters are used to produce mRNAs for soluble and membrane-bound forms of the enzyme. DNA Cell Biol. 1993;12:253–263. - PubMed
-
- Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry. 2006;60:141–151. - PubMed
-
- Hayden EP, Nurnberger JI., Jr Molecular genetics of bipolar disorder. Genes Brain Behav. 2006;5:85–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

