Enhanced survival of spiral ganglion cells after cessation of treatment with brain-derived neurotrophic factor in deafened guinea pigs
- PMID: 19365690
- PMCID: PMC2717388
- DOI: 10.1007/s10162-009-0170-2
Enhanced survival of spiral ganglion cells after cessation of treatment with brain-derived neurotrophic factor in deafened guinea pigs
Abstract
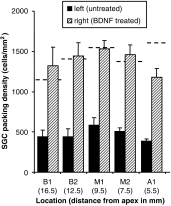
Exogenous delivery of neurotrophic factors into the cochlea of deafened animals rescues spiral ganglion cells (SGCs) from degeneration. To be clinically relevant for human cochlear implant candidates, the protective effect of neurotrophins should persist after cessation of treatment and the treated SGCs should remain functional. In this study, the survival and functionality of SGCs were investigated after temporary treatment with brain-derived neurotrophic factor (BDNF). Guinea pigs in the experimental group were deafened, and 2 weeks later, the right cochleae were implanted with an electrode array and drug delivery cannula. BDNF was administered to the implanted cochleae during a 4-week period via a mini-osmotic pump. After completion of the treatment, the osmotic pumps were removed. Two weeks later, the animals were killed and the survival of SGCs was analyzed. To monitor the functionality of the auditory nerve, electrically evoked auditory brainstem responses (eABRs) were recorded in awake animals throughout the experiment. BDNF treatment resulted in enhanced survival of SGCs 2 weeks after cessation of the treatment and prevented the decreases in size and circularity that are seen in the untreated contralateral cochleae. The amplitude of the suprathreshold eABR response in BDNF-treated animals was significantly larger than in deafened control animals and comparable to that in normal-hearing control animals. The amplitude in the BDNF-treated group did not decrease significantly after cessation of treatment. The eABR latency in BDNF-treated animals was longer than normal and comparable to that in deafened control animals. These morphological and functional findings demonstrate that neurotrophic intervention had a lasting effect, which is promising for future clinical application of neurotrophic factors in implanted human cochleae.
Figures









References
-
- Blamey P. Are spiral ganglion cell numbers important for speech perception with a cochlear implant? Am. J. Otol. 1997;18(suppl):11–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

