Altered somatosensory processing in trigeminal neuralgia
- PMID: 19365802
- PMCID: PMC6871034
- DOI: 10.1002/hbm.20773
Altered somatosensory processing in trigeminal neuralgia
Abstract
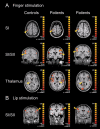
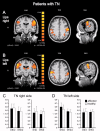
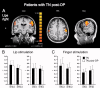
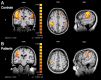
Trigeminal neuralgia (TN) is a pain state characterized by intermittent unilateral pain attacks in one or several facial areas innervated by the trigeminal nerve. The somatosensory cortex is heavily involved in the perception of sensory features of pain, but it is also the primary target for thalamic input of nonpainful somatosensory information. Thus, pain and somatosensory processing are accomplished in overlapping cortical structures raising the question whether pain states are associated with alteration of somatosensory function itself. To test this hypothesis, we used functional magnetic resonance imaging to assess activation of primary (SI) and secondary (SII) somatosensory cortices upon nonpainful tactile stimulation of lips and fingers in 18 patients with TN and 10 patients with TN relieved from pain after successful neurosurgical intervention in comparison with 13 healthy subjects. We found that SI and SII activations in patients did neither depend on the affected side of TN nor differ between operated and nonoperated patients. However, SI and SII activations, but not thalamic activations, were significantly reduced in patients as compared to controls. These differences were most prominent for finger stimulation, an area not associated with TN. For lip stimulation SI and SII activations were reduced in patients with TN on the contra- but not on the ipsilateral side to the stimulus. These findings suggest a general reduction of SI and SII processing in patients with TN, indicating a long-term modulation of somatosensory function and pointing to an attempt of cortical adaptation to potentially painful stimuli.
Figures


References
-
- Annett M ( 1970): A classification of hand preference by association analysis. Br J Psychol 61: 303–321. - PubMed
-
- Apkarian AV,Bushnell MC,Treede RD,Zubieta JK ( 2005): Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9: 463–484. - PubMed
-
- Barker FG II,Jannetta PJ,Bissonette DJ,Larkins MV,Jho HD ( 1996): The long‐term outcome of microvascular decompression for trigeminal neuralgia. N Engl J Med 334: 1077–1083. - PubMed
-
- Basbaum AI,Jessell TM ( 2000): The perception of pain In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. New York: McGraw‐Hill; pp 472–491.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

