Design and synthesis of a library of tetracyclic hydroazulenoisoindoles
- PMID: 19366169
- PMCID: PMC3104412
- DOI: 10.1021/cc900024p
Design and synthesis of a library of tetracyclic hydroazulenoisoindoles
Abstract
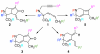
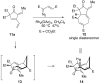
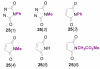
Forty-four tetracyclic hydroazulenoisoindoles were synthesized via a tandem cyclopropanation/Cope rearrangement, followed by a Diels-Alder sequence from easily available five-membered cyclic cross-conjugated trienones. These trienones were obtained from two different routes depending upon whether R(1) and R(2) are alkyl or amino acid derived functional groups, via a rhodium(I)-catalyzed cycloisomerization reaction. To increase diversity, four maleimides and two 1,2,4-triazoline-3,5-diones were used as dienophiles in the Diels-Alder step. Several Diels-Alder adducts were further reacted under palladium-catalyzed hydrogenation conditions, leading to a diastereoselective reduction of the trisubstituted double bond. This library has demonstrated rapid access to a variety of structurally complex natural product-like compounds via stereochemical diversity and building block diversity approaches.
Figures
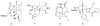
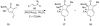
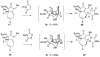
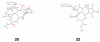
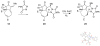



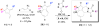
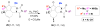
References
-
- Sauer WHB, Schwarz MK. J. Chem. Inf. Comput. Sci. 2003;43:987–1003. - PubMed
-
- Lipkus AH, Yuan Q, Lucas KA, Funk SA, Bartelt WF, III, Schenck RJ, Trippe AJ. J. Org. Chem. 2008;73:4443–4451. - PubMed
-
-
For a review on a DOS planning strategy, see: Burke MD, Schreiber SL. Angew. Chem. Int. Ed. 2004;43:46–58.
-
-
- Lipinski C, Hopkins A. Nature. 2004. pp. 855–861. The NIH Roadmap http://www.nihroadmap.nih.gov. - PubMed
-
- Brummond KM, Mitasev B. Org. Lett. 2004;6:2245–2248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

