Chemoselective tryptophan labeling with rhodium carbenoids at mild pH
- PMID: 19366262
- PMCID: PMC2722835
- DOI: 10.1021/ja900094h
Chemoselective tryptophan labeling with rhodium carbenoids at mild pH
Abstract
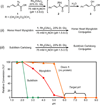
Significant improvements have been made to a previously reported tryptophan modification method using rhodium carbenoids in aqueous solution, allowing the reaction to proceed at pH 6-7. This technique is based on the discovery that N-(tert-butyl)hydroxylamine promotes indole modification with rhodium carbenoids over a broad pH range (2-7). This methodology was demonstrated on peptide and protein substrates, generally yielding 40-60% conversion with excellent tryptophan chemoselectivity. The solvent accessibility of the indole side chains was found to be a key factor in successful carbenoid addition, as demonstrated by conducting the reaction at temperatures high enough to cause thermal denaturation of the protein substrate. Progress toward the expression of proteins bearing solvent accessible tryptophan residues as reactive handles for modification with rhodium carbenoids is also reported.
Figures






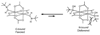



References
-
- Hermanson GT. Bioconjugate Techniques. 1 ed. San Diego: Academic Press; 1996.
-
- Tilley SD, Joshi NS, Francis MB. Chemistry and Chemical Reactivity of Proteins. In: Begley TP, editor. Wiley Encyclopedia of Chemical Biology. Hoboken, NJ: Wiley; 2008. in press.
-
- Koshland DE, Karkhanis YD, Latham HG. J. Am. Chem. Soc. 1964;86:1448–1450.
-
- Savige WE, Fontana A. Methods in Enzymology. 1977;47:442–453. - PubMed
-
- Antos JM, Francis MB. J. Am. Chem. Soc. 2004;126:10256–10257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

