Pentameric concatenated (alpha4)(2)(beta2)(3) and (alpha4)(3)(beta2)(2) nicotinic acetylcholine receptors: subunit arrangement determines functional expression
- PMID: 19366353
- PMCID: PMC2697715
- DOI: 10.1111/j.1476-5381.2008.00104.x
Pentameric concatenated (alpha4)(2)(beta2)(3) and (alpha4)(3)(beta2)(2) nicotinic acetylcholine receptors: subunit arrangement determines functional expression
Abstract
Background and purpose: alpha4 and beta2 nicotinic acetylcholine (ACh) receptor subunits expressed heterologously in Xenopus oocytes assemble into a mixed population of (alpha4)(2)(beta2)(3) and (alpha4)(3)(beta2)(2) receptors. In order to express these receptors separately in heterologous systems, we have engineered pentameric concatenated (alpha4)(2)(beta2)(3) and (alpha4)(3)(beta2)(2) receptors.
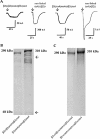
Experimental approach: alpha4 and beta2 subunits were concatenated by synthetic linkers into pentameric constructs to produce either (alpha4)(2)(beta2)(3) or (alpha4)(3)(beta2)(2) receptors. Using two-electrode voltage-clamp techniques, we examined the ability of the concatenated constructs to produce functional expression in Xenopus oocytes. Functional constructs were further characterized in respect to agonists, competitive antagonists, Ca2+ permeability, sensitivity to modulation by Zn2+ and sensitivity to up-regulation by chaperone protein 14-3-3.
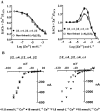
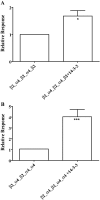
Key results: We found that pentameric concatamers with a subunit arrangement of beta2_alpha4_beta2_alpha4_beta2 or beta2_alpha4_beta2_alpha4_alpha4 were stable and functional in Xenopus oocytes. By comparison, when alpha4 and beta2 were concatenated with a subunit order of beta2_beta2_alpha4_beta2_alpha4 or beta2_alpha4_alpha4_beta2_alpha4, functional expression in Xenopus oocytes was very low, even though the proteins were synthesized and stable. Both beta2_alpha4_beta2_alpha4_beta2 and beta2_alpha4_beta2_alpha4_alpha4 concatamers recapitulated the ACh concentration response curve, the sensitivity to Zn2+ modulation, Ca2+ permeability and the sensitivity to up-regulation by chaperone protein 14-3-3 of the corresponding non-linked (alpha4)(2)(beta2)(3) and (alpha4)(3)(beta2)(2) receptors respectively. Using these concatamers, we found that most alpha4beta2-preferring compounds studied, including A85380, 5I-A85380, cytisine, epibatidine, TC2559 and dihydro-beta-erythroidine, demonstrate stoichiometry-specific potencies and efficacies.
Conclusions and implications: We concluded that the alpha4beta2 nicotinic ACh receptors produced with beta2_alpha4_beta2_alpha4_beta2 or beta2_alpha4_beta2_alpha4_alpha4 pentameric constructs are valid models of non-linked (alpha4)(2)(beta2)(3) and (alpha4)(3)(beta2)(2) receptors respectively.
Figures



References
-
- Baur R, Minier F, Sigel E. A GABAA receptor of defined subunit composition and positioning: concatenation of five subunits. FEBS Lett. 2006;580:1616–1620. - PubMed
-
- Brejc K, van Dijk WJ, Klaassen RV, Schuurman M, van Der Oost J, Smit AB, et al. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. - PubMed
-
- Butler AS, Lindsey SA, Dover TJ, Kennedy MD, Hope AG, Barnes NM. The importance of the C-terminus of the human 5-HT3A receptor subunit. Neuropharmacology. doi: 10.1016/j.neuropharm.2008.08.017 [Epub ahead of print. - PubMed
-
- Butt CM, Hutton SR, Marks MJ, Collins AC. Bovine serum albumin enhances nicotinic acetylcholine receptor function in mouse thalamic synaptosomes. J Neurochem. 2002;83:48–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

