A modelled economic evaluation comparing atomoxetine with methylphenidate in the treatment of children with attention-deficit/hyperactivity disorder in Spain
- PMID: 19366449
- PMCID: PMC2674033
- DOI: 10.1186/1471-244X-9-15
A modelled economic evaluation comparing atomoxetine with methylphenidate in the treatment of children with attention-deficit/hyperactivity disorder in Spain
Abstract
Background: Attention Deficit/Hyperactivity Disorder (ADHD) is a neurobehavioural disorder, affecting 3-6% of school age children and adolescents in Spain. Methylphenidate (MPH), a mild stimulant, had long been the only approved medication available for ADHD children in Spain. Atomoxetine is a non-stimulant alternative in the treatment of ADHD with once-a-day oral dosing. This study aims to estimate the cost-effectiveness of atomoxetine compared to MPH. In addition, atomoxetine is compared to 'no medication' for patient populations who are ineligible for MPH (i.e. having stimulant-failure experience or co-morbidities precluding stimulant medication).
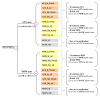
Methods: An economic model with Markov processes was developed to estimate the costs and benefits of atomoxetine versus either MPH or 'no medication'. The incremental cost per quality-adjusted life-year (QALY) was calculated for atomoxetine relative to the comparators. The Markov process incorporated 14 health states, representing a range of outcomes associated with treatment options. Utility values were obtained from the utility valuation survey of 83 parents of children with ADHD. The clinical data were based on a thorough review of controlled clinical trials and other clinical literature, and validated by international experts. Costs and outcomes were estimated using Monte Carlo simulation over a 1-year duration, with costs estimated from the perspective of the National Health Service in Spain.
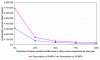
Results: For stimulant-naive patients without contra-indications to stimulants, the incremental costs per QALY gained for atomoxetine were euro 34,308 (compared to an immediate-release MPH) and euro 24,310 (compared to an extended-release MPH). For those patients who have stimulant-failure experience or contra-indications to stimulants, the incremental costs per QALY gained of atomoxetine compared to 'no medication' were euro 23,820 and euro 23,323, respectively.
Conclusion: The economic evaluation showed that atomoxetine is an effective alternative across a range of ADHD populations and offers value-for money in the treatment of ADHD.
Figures
References
-
- Jensen PS, Garcia JA, Glied S, Crowe M, Foster M, Schlander M, Hinshaw S, Vitiello B, Arnold LE, Elliott G, et al. Cost-effectiveness of ADHD treatments: findings from the multimodal treatment study of children with ADHD. Am J Psychiatry. 2005;162:1628–1636. doi: 10.1176/appi.ajp.162.9.1628. - DOI - PubMed
-
- Biederman J. Attention-deficit/hyperactivity disorder: a life-span perspective. J Clin Psychiatry. 1998;59:4–16. - PubMed
-
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press; 2000.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

