Daily rhythms of food-anticipatory behavioral activity do not require the known circadian clock
- PMID: 19366674
- PMCID: PMC2666092
- DOI: 10.1073/pnas.0902063106
Daily rhythms of food-anticipatory behavioral activity do not require the known circadian clock
Abstract
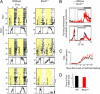
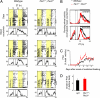
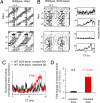
When food availability is restricted to a particular time each day, mammals exhibit food-anticipatory activity (FAA), a daily increase in locomotor activity preceding the presentation of food. Considerable historical evidence suggests that FAA is driven by a food-entrainable circadian clock distinct from the master clock of the suprachiasmatic nucleus. Multiple food-entrainable circadian clocks have been discovered in the brain and periphery, raising strong expectations that one or more underlie FAA. We report here that mutant mice lacking known circadian clock function in all tissues exhibit normal FAA both in a light-dark cycle and in constant darkness, regardless of whether the mutation disables the positive or negative limb of the clock feedback mechanism. FAA is thus independent of the known circadian clock. Our results indicate either that FAA is not the output of an oscillator or that it is the output of a circadian oscillator different from known circadian clocks.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Mistlberger RE. Circadian food-anticipatory activity: Formal models and physiological mechanisms. Neurosci Biobehav Rev. 1994;18:171–195. - PubMed
-
- Stephan FK. Food entrainable oscillators in mammals. In: Takahashi JS, Turek FW, Moore RY, editors. Circadian Clocks, Handbook of Behavioral Neurobiology. Vol 12. New York: Kluwer Academic/Plenum; 2001. pp. 223–246.
-
- Pittendrigh CS. Circadian systems: General perspective. In: Aschoff J, editor. Biological Rhythms, Handbook of Behavioral Neurobiology. Vol 4. New York: Kluwer Academic/Plenum; 1981. pp. 57–80.
-
- Coleman GJ, Harper S, Clarke JD Armstrong S. Evidence for a separate meal-associated oscillator in the rat. Physiol Behav. 1982;29:107–115. - PubMed
-
- Stephan FK, Swann JM, Sisk CL. Anticipation of 24-hr feeding schedules in rats with lesions of the suprachiasmatic nucleus. Behav Neural Biol. 1979;25:346–363. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

