Bone morphogenetic protein (BMP) and activin type II receptors balance BMP9 signals mediated by activin receptor-like kinase-1 in human pulmonary artery endothelial cells
- PMID: 19366699
- PMCID: PMC2708876
- DOI: 10.1074/jbc.M109.002881
Bone morphogenetic protein (BMP) and activin type II receptors balance BMP9 signals mediated by activin receptor-like kinase-1 in human pulmonary artery endothelial cells
Abstract
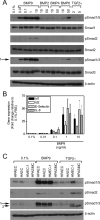
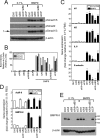
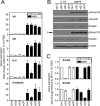
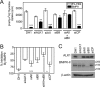
Mutations in transforming growth factor-beta (TGF-beta) receptor superfamily members underlie conditions characterized by vascular dysplasia. Mutations in endoglin and activin-like kinase receptor 1 (ALK1) cause hereditary hemorrhagic telangiectasia, whereas bone morphogenetic protein type II receptor (BMPR-II) mutations underlie familial pulmonary arterial hypertension. To understand the functional roles of these receptors, we examined their relative contributions to BMP signaling in human pulmonary artery endothelial cells (HPAECs). BMP9 potently and selectively induced Smad1/5 phosphorylation and Id gene expression in HPAECs. Contrary to expectations, BMP9 also stimulated Smad2 activation. Furthermore, BMP9 induced the expression of interleukin 8 and E-selectin. Using small interfering RNA, we demonstrate that the type I receptor, ALK1, is essential for these responses. However, small interfering RNA and inhibitor studies showed no involvement of ALK5 or endoglin. We further demonstrate that, of the candidate type II receptors, BMPR-II predominantly mediated IL-8 and E-selectin induction and mitogenic inhibition by BMP9. Conversely, activin receptor type II (ActR-II) contributed more to BMP9-mediated Smad2 activation. Only abolition of both type II receptors significantly reduced the Smad1/5 and Id responses. Both ALK1 and BMPR-II contributed to growth inhibition of HPAECs, whereas ActR-II was not involved. Taken together, our findings demonstrate the critical role of type II receptors in balancing BMP9 signaling via ALK1 and emphasize the essential role for BMPR-II in a subset of BMP9 responses (interleukin 8, E-selectin, and proliferation). This differential signaling may contribute to the contrasting pathologies of hereditary hemorrhagic telangiectasia and pulmonary arterial hypertension.
Figures



References
-
- Lane K. B., Machado R. D., Pauciulo M. W., Thomson J. R., Phillips J. A., 3rd, Loyd J. E., Nichols W. C., Trembath R. C. ( 2000) Nat. Genet. 26, 81– 84 - PubMed
-
- Machado R. D., Aldred M. A., James V., Harrison R. E., Patel B., Schwalbe E. C., Gruenig E., Janssen B., Koehler R., Seeger W., Eickelberg O., Olschewski H., Elliott C. G., Glissmeyer E., Carlquist J., Kim M., Torbicki A., Fijalkowska A., Szewczyk G., Parma J., Abramowicz M. J., Galie N., Morisaki H., Kyotani S., Nakanishi N., Morisaki T., Humbert M., Simonneau G., Sitbon O., Soubrier F., Coulet F., Morrell N. W., Trembath R. C. ( 2006) Hum. Mutat. 27, 121– 132 - PubMed
-
- Sadick H., Sadick M., Götte K., Naim R., Riedel F., Bran G., Hörmann K. ( 2006) Wien. Klin. Wochenschr. 118, 72– 80 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

