Recruitment of vimentin to the cell surface by beta3 integrin and plectin mediates adhesion strength
- PMID: 19366731
- PMCID: PMC2721003
- DOI: 10.1242/jcs.043042
Recruitment of vimentin to the cell surface by beta3 integrin and plectin mediates adhesion strength
Abstract
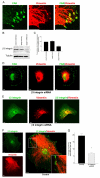
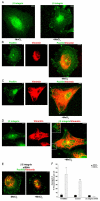
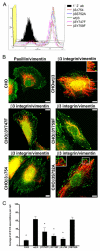
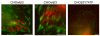
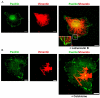
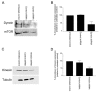
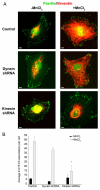
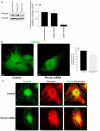
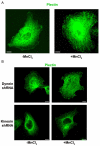
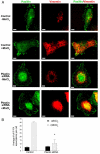
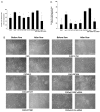
Much effort has been expended on analyzing how microfilament and microtubule cytoskeletons dictate the interaction of cells with matrix at adhesive sites called focal adhesions (FAs). However, vimentin intermediate filaments (IFs) also associate with the cell surface at FAs in endothelial cells. Here, we show that IF recruitment to FAs in endothelial cells requires beta3 integrin, plectin and the microtubule cytoskeleton, and is dependent on microtubule motors. In CHO cells, which lack beta3 integrin but contain vimentin, IFs appear to be collapsed around the nucleus, whereas in CHO cells expressing beta3 integrin (CHOwtbeta3), vimentin IFs extend to FAs at the cell periphery. This recruitment is regulated by tyrosine residues in the beta3 integrin cytoplasmic tail. Moreover, CHOwtbeta3 cells exhibit significantly greater adhesive strength than CHO or CHO cells expressing mutated beta3 integrin proteins. These differences require an intact vimentin network. Therefore, vimentin IF recruitment to the cell surface is tightly regulated and modulates the strength of adhesion of cells to their substrate.
Figures
References
-
- Axelrod, D. (1989). Total internal reflection fluorescence microscopy. Methods Cell Biol. 30, 245-270. - PubMed
-
- Bershadsky, A. D., Tint, I. S. and Svitkina, T. M. (1987). Association of intermediate filaments with vinculin-containing adhesion plaques of fibroblasts. Cell Motil. Cytoskeleton 8, 274-283. - PubMed
-
- Blystone, S. D. (2004). Integrating an integrin: a direct route to actin. Biochim. Biophys. Acta 1692, 47-54. - PubMed
-
- Blystone, S. D., Lindberg, F. P., Williams, M. P., McHugh, K. P. and Brown, E. J. (1996). Inducible tyrosine phosphorylation of the β3 integrin requires the αv integrin cytoplasmic tail. J. Biol. Chem. 271, 31458-31462. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

