PIK3CA and PIK3CB inhibition produce synthetic lethality when combined with estrogen deprivation in estrogen receptor-positive breast cancer
- PMID: 19366795
- PMCID: PMC2811393
- DOI: 10.1158/0008-5472.CAN-08-4450
PIK3CA and PIK3CB inhibition produce synthetic lethality when combined with estrogen deprivation in estrogen receptor-positive breast cancer
Abstract
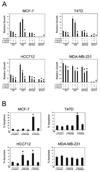
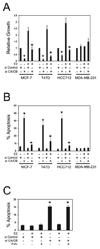
Several phosphoinositide 3-kinase (PI3K) catalytic subunit inhibitors are currently in clinical trial. We therefore sought to examine relationships between pharmacologic inhibition and somatic mutations in PI3K catalytic subunits in estrogen receptor (ER)-positive breast cancer, in which these mutations are particularly common. RNA interference (RNAi) was used to determine the effect of selective inhibition of PI3K catalytic subunits, p110alpha and p110beta, in ER(+) breast cancer cells harboring either mutation (PIK3CA) or gene amplification (PIK3CB). p110alpha RNAi inhibited growth and promoted apoptosis in all tested ER(+) breast cancer cells under estrogen deprived-conditions, whereas p110beta RNAi only affected cells harboring PIK3CB amplification. Moreover, dual p110alpha/p110beta inhibition potentiated these effects. In addition, treatment with the clinical-grade PI3K catalytic subunit inhibitor BEZ235 also promoted apoptosis in ER(+) breast cancer cells. Importantly, estradiol suppressed apoptosis induced by both gene knockdowns and BEZ235 treatment. Our results suggest that PI3K inhibitors should target both p110alpha and p110beta catalytic subunits, whether wild-type or mutant, and be combined with endocrine therapy for maximal efficacy when treating ER(+) breast cancer.
Conflict of interest statement
Conflicts of Interest: No pharmaceutical company funding was received for this research project. Dr Ellis has received honoraria, grants and has served as a consultant for AstraZenica, Novartis and Pfizer.
Figures



References
-
- EBCTCG. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. - PubMed
-
- Dowsett M, Smith IE, Ebbs SR, et al. Proliferation and apoptosis as markers of benefit in neoadjuvant endocrine therapy of breast cancer. Clin Cancer Res. 2006;12:1024s–1030s. - PubMed
-
- Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. - PubMed
-
- Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

