14-3-3 and its binding partners are regulators of protein-protein interactions during spermatogenesis
- PMID: 19366886
- PMCID: PMC2804912
- DOI: 10.1677/JOE-09-0041
14-3-3 and its binding partners are regulators of protein-protein interactions during spermatogenesis
Abstract
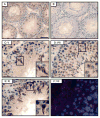
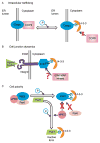
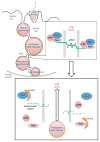
During spermatogenesis, spermiation takes place at the adluminal edge of the seminiferous epithelium at stage VIII of the epithelial cycle during which fully developed spermatids (i.e. spermatozoa) detach from the epithelium in adult rat testes. This event coincides with the migration of preleptotene/leptotene spermatocytes across the blood-testis barrier from the basal to the apical (or adluminal) compartment. At stage XIV of the epithelial cycle, Pachytene spermatocytes (diploid, 2n) differentiate into diplotene spermatocytes (tetraploid, 4n) in the apical compartment of the epithelium, which begin meiosis I to be followed by meiosis II to form spermatids (haploid, 1n) at stage XIV of the epithelial cycle. These spermatids, in turn, undergo extensive morphological changes and traverse the seminiferous epithelium until they differentiate into elongated spermatids. Thus, there are extensive changes at the Sertoli-Sertoli and Sertoli-germ cell interface via protein 'coupling' and 'uncoupling' between cell adhesion protein complexes, as well as changes in interactions between integral membrane proteins and their peripheral adaptors, regulatory protein kinases and phosphatases, and the cytoskeletal proteins. These precisely coordinated protein-protein interactions affect cell adhesion and cell movement. In this review, we focus on the 14-3-3 protein family, whose members have different binding partners in the seminiferous epithelium. Recent studies have illustrated that 14-3-3 affects protein-protein interactions in the seminiferous epithelium, and regulates cell adhesion possibly via its effects on intracellular protein trafficking and cell-polarity proteins. This review provides a summary on the latest findings regarding the role of 14-3-3 family of proteins and their potential implications on spermatogenesis. We also highlight research areas that deserve attentions by investigators.
Conflict of interest statement
The authors herein declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research/work reported.
Figures
Similar articles
-
Extracellular matrix: recent advances on its role in junction dynamics in the seminiferous epithelium during spermatogenesis.Biol Reprod. 2004 Aug;71(2):375-91. doi: 10.1095/biolreprod.104.028225. Epub 2004 Apr 28. Biol Reprod. 2004. PMID: 15115723 Review.
-
Intercellular adhesion molecules (ICAMs) and spermatogenesis.Hum Reprod Update. 2013 Mar-Apr;19(2):167-86. doi: 10.1093/humupd/dms049. Epub 2013 Jan 3. Hum Reprod Update. 2013. PMID: 23287428 Free PMC article. Review.
-
Transport of germ cells across the seminiferous epithelium during spermatogenesis-the involvement of both actin- and microtubule-based cytoskeletons.Tissue Barriers. 2016 Nov 28;4(4):e1265042. doi: 10.1080/21688370.2016.1265042. eCollection 2016. Tissue Barriers. 2016. PMID: 28123928 Free PMC article. Review.
-
Disruption of Sertoli-germ cell adhesion function in the seminiferous epithelium of the rat testis can be limited to adherens junctions without affecting the blood-testis barrier integrity: an in vivo study using an androgen suppression model.J Cell Physiol. 2005 Oct;205(1):141-57. doi: 10.1002/jcp.20377. J Cell Physiol. 2005. PMID: 15880438
-
Cell polarity proteins and spermatogenesis.Semin Cell Dev Biol. 2016 Nov;59:62-70. doi: 10.1016/j.semcdb.2016.06.008. Epub 2016 Jun 9. Semin Cell Dev Biol. 2016. PMID: 27292315 Free PMC article. Review.
Cited by
-
Regulation of blood-testis barrier (BTB) dynamics during spermatogenesis via the "Yin" and "Yang" effects of mammalian target of rapamycin complex 1 (mTORC1) and mTORC2.Int Rev Cell Mol Biol. 2013;301:291-358. doi: 10.1016/B978-0-12-407704-1.00006-3. Int Rev Cell Mol Biol. 2013. PMID: 23317821 Free PMC article. Review.
-
Regulation of actin dynamics and protein trafficking during spermatogenesis--insights into a complex process.Crit Rev Biochem Mol Biol. 2013 Mar-Apr;48(2):153-72. doi: 10.3109/10409238.2012.758084. Epub 2013 Jan 23. Crit Rev Biochem Mol Biol. 2013. PMID: 23339542 Free PMC article. Review.
-
Identification of flucloxacillin-modified hepatocellular proteins: implications in flucloxacillin-induced liver injury.Toxicol Sci. 2023 Mar 20;192(1):106-116. doi: 10.1093/toxsci/kfad015. Toxicol Sci. 2023. PMID: 36782357 Free PMC article.
-
Spermiation: The process of sperm release.Spermatogenesis. 2011 Jan;1(1):14-35. doi: 10.4161/spmg.1.1.14525. Spermatogenesis. 2011. PMID: 21866274 Free PMC article.
-
14-3-3 Protein regulates cell adhesion in the seminiferous epithelium of rat testes.Endocrinology. 2009 Oct;150(10):4713-23. doi: 10.1210/en.2009-0427. Epub 2009 Jul 16. Endocrinology. 2009. PMID: 19608648 Free PMC article.
References
-
- Aitken A. 14-3-3 Proteins: a historic overview. Seminars in Cancer Biology. 2006;16:162–172. - PubMed
-
- Anderson H, Bergstralh D, Kawamura T, Blauvelt A, Roche P. Phosphorylation of the invariant chain by protein kinase C regulates MHC Class II trafficking to antigen-processing compartments. Journal of Immunology. 1999;163:5435–5443. - PubMed
-
- Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochimica et Biophysica Acta. 2007;1778:614–630. - PubMed
-
- Barlowe C. Signals for COPII-dependent export from the ER: what’s the ticket out? Trends in Cell Biology. 2003;13:295–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

