Evidence that the swim afferent neurons of tritonia diomedea are glutamatergic
- PMID: 19366921
- PMCID: PMC3073080
- DOI: 10.1086/BBLv216n2p103
Evidence that the swim afferent neurons of tritonia diomedea are glutamatergic
Abstract
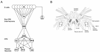
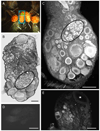
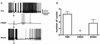
The escape swim response of the marine mollusc Tritonia diomedea is a well-established model system for studies of the neural basis of behavior. Although the swim neural network is reasonably well understood, little is known about the transmitters used by its constituent neurons. In the present study, we provide immunocytochemical and electrophysiological evidence that the S-cells, the afferent neurons that detect aversive skin stimuli and in turn trigger Tritonia's escape swim response, use glutamate as their transmitter. First, immunolabeling revealed that S-cell somata contain elevated levels of glutamate compared to most other neurons in the Tritonia brain, consistent with findings from glutamatergic neurons in many species. Second, pressure-applied puffs of glutamate produced the same excitatory response in the target neurons of the S-cells as the naturally released S-cell transmitter itself. Third, the glutamate receptor antagonist CNQX completely blocked S-cell synaptic connections. These findings support glutamate as a transmitter used by the S-cells, and will facilitate studies using this model system to explore a variety of issues related to the neural basis of behavior.
Figures



Similar articles
-
Immunochemical and electrophysiological analyses of magnetically responsive neurons in the mollusc Tritonia diomedea.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006 Mar;192(3):235-45. doi: 10.1007/s00359-005-0063-8. Epub 2005 Oct 21. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006. PMID: 16240147
-
Long-term habituation in the marine mollusc Tritonia diomedea.Biol Bull. 2006 Jun;210(3):230-7. doi: 10.2307/4134560. Biol Bull. 2006. PMID: 16801497
-
Dishabituation of the Tritonia escape swim.Learn Mem. 2000 Jan;7(1):43-7. doi: 10.1101/lm.7.1.43. Learn Mem. 2000. PMID: 10706601 Free PMC article.
-
Neuromodulation intrinsic to the central pattern generator for escape swimming in Tritonia.Ann N Y Acad Sci. 1998 Nov 16;860:181-8. doi: 10.1111/j.1749-6632.1998.tb09048.x. Ann N Y Acad Sci. 1998. PMID: 9928311 Review.
-
The legacies of A. O. Dennis Willows and Peter A. Getting: neuroscience research using Tritonia.J Neurophysiol. 2025 Jan 1;133(1):34-45. doi: 10.1152/jn.00318.2024. Epub 2024 Nov 29. J Neurophysiol. 2025. PMID: 39611858 Review.
Cited by
-
Aplysia Ganglia preparation for electrophysiological and molecular analyses of single neurons.J Vis Exp. 2014 Jan 13;(83):e51075. doi: 10.3791/51075. J Vis Exp. 2014. PMID: 24457225 Free PMC article.
-
Cellular-resolution gene expression mapping reveals organization in the head ganglia of the gastropod, Berghia stephanieae.J Comp Neurol. 2024 Jun;532(6):e25628. doi: 10.1002/cne.25628. J Comp Neurol. 2024. PMID: 38852042 Free PMC article.
-
Functional Characterization of a Vesicular Glutamate Transporter in an Interneuron That Makes Excitatory and Inhibitory Synaptic Connections in a Molluscan Neural Circuit.J Neurosci. 2015 Jun 17;35(24):9137-49. doi: 10.1523/JNEUROSCI.0180-15.2015. J Neurosci. 2015. PMID: 26085636 Free PMC article.
-
Axonal conduction block as a novel mechanism of prepulse inhibition.J Neurosci. 2012 Oct 31;32(44):15262-70. doi: 10.1523/JNEUROSCI.0160-12.2012. J Neurosci. 2012. PMID: 23115164 Free PMC article.
-
Neural shutdown under stress: an evolutionary perspective on spreading depolarization.J Neurophysiol. 2020 Mar 1;123(3):885-895. doi: 10.1152/jn.00724.2019. Epub 2020 Feb 5. J Neurophysiol. 2020. PMID: 32023142 Free PMC article. Review.
References
-
- Antonov I, Antonova I, Kandel ER, Hawkins RD. Activity-dependent presynaptic facilitation and hebbian LTP are both required and interact during classical conditioning in Aplysia. Neuron. 2003;37:135–147. - PubMed
-
- Antzoulatos EG, Byrne JH. Learning insights transmitted by glutamate. Trends Neurosci. 2004;27:555–560. - PubMed
-
- Baccus SA, Burrell BD, Sahley CL, Muller KJ. Action potential reflection and failure at axon branch points cause stepwise changes in EPSPs in a neuron essential for learning. J. Neurophysiol. 2000;83:1693–1700. - PubMed
-
- Beck JC, Cooper MS, Willows AO. Immunocytochemical localization of pedal peptide in the central nervous system of the gastropod mollusc Tritonia diomedea. J. Comp. Neurol. 2000;425:1–9. - PubMed
-
- Bravarenko NI, Korshunova TA, Malyshev AY, Balaban PM. Synaptic contact between mechanosensory neuron and withdrawal interneuron in terrestrial snail is mediated by L-glutamate-like transmitter. Neurosci. Lett. 2003;341:237–240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

