Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes
- PMID: 19366986
- PMCID: PMC2699232
- DOI: 10.1182/blood-2008-12-195354
Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes
Abstract
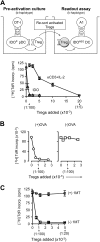
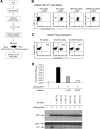
The immunoregulatory enzyme indoleamine 2,3-dioxygenase (IDO) is expressed by a subset of murine plasmacytoid DCs (pDCs) in tumor-draining lymph nodes (TDLNs), where it can potently activate Foxp3+ regulatory T cells (Tregs). We now show that IDO functions as a molecular switch in TDLNs, maintaining Tregs in their normal suppressive phenotype when IDO was active, but allowing inflammation-induced conversion of Tregs to a polyfunctional T-helper phenotype similar to proinflammatory T-helper-17 (TH17) cells when IDO was blocked. In vitro, conversion of Tregs to the TH17-like phenotype was driven by antigen-activated effector T cells and required interleukin-6 (IL-6) produced by activated pDCs. IDO regulated this conversion by dominantly suppressing production of IL-6 in pDCs, in a GCN2-kinase dependent fashion. In vivo, using a model of established B16 melanoma, the combination of an IDO-inhibitor drug plus antitumor vaccine caused up-regulation of IL-6 in pDCs and in situ conversion of a majority of Tregs to the TH17 phenotype, with marked enhancement of CD8+ T-cell activation and antitumor efficacy. Thus, Tregs in TDLNs can be actively reprogrammed in situ into T-helper cells, without the need for physical depletion, and IDO serves as a key regulator of this critical conversion.
Figures





Similar articles
-
Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase.J Clin Invest. 2007 Sep;117(9):2570-82. doi: 10.1172/JCI31911. J Clin Invest. 2007. PMID: 17710230 Free PMC article.
-
IDO activates regulatory T cells and blocks their conversion into Th17-like T cells.J Immunol. 2009 Aug 15;183(4):2475-83. doi: 10.4049/jimmunol.0900986. Epub 2009 Jul 27. J Immunol. 2009. PMID: 19635913 Free PMC article.
-
Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes.J Clin Invest. 2004 Jul;114(2):280-90. doi: 10.1172/JCI21583. J Clin Invest. 2004. PMID: 15254595 Free PMC article.
-
IL-17 and Th17 Cells.Annu Rev Immunol. 2009;27:485-517. doi: 10.1146/annurev.immunol.021908.132710. Annu Rev Immunol. 2009. PMID: 19132915 Review.
-
T cell regulatory plasmacytoid dendritic cells expressing indoleamine 2,3 dioxygenase.Handb Exp Pharmacol. 2009;(188):165-96. doi: 10.1007/978-3-540-71029-5_8. Handb Exp Pharmacol. 2009. PMID: 19031026 Free PMC article. Review.
Cited by
-
Immunometabolism at the Nexus of Cancer Therapeutic Efficacy and Resistance.Front Immunol. 2021 May 17;12:657293. doi: 10.3389/fimmu.2021.657293. eCollection 2021. Front Immunol. 2021. PMID: 34079545 Free PMC article. Review.
-
Altered tryptophan metabolism as a paradigm for good and bad aspects of immune privilege in chronic inflammatory diseases.Front Immunol. 2012 May 11;3:109. doi: 10.3389/fimmu.2012.00109. eCollection 2012. Front Immunol. 2012. PMID: 22593757 Free PMC article.
-
IFN-γ and indoleamine 2,3-dioxygenase signaling between donor dendritic cells and T cells regulates graft versus host and graft versus leukemia activity.Blood. 2012 Jan 26;119(4):1075-85. doi: 10.1182/blood-2010-12-322891. Epub 2011 Nov 30. Blood. 2012. PMID: 22130799 Free PMC article.
-
How Changes in the Nutritional Landscape Shape Gut Immunometabolism.Nutrients. 2021 Mar 2;13(3):823. doi: 10.3390/nu13030823. Nutrients. 2021. PMID: 33801480 Free PMC article. Review.
-
Plasmacytoid dendritic cells in HIV infection.Adv Exp Med Biol. 2013;762:71-107. doi: 10.1007/978-1-4614-4433-6_3. Adv Exp Med Biol. 2013. PMID: 22975872 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

