Ontogeny and nutritional control of adipogenesis in zebrafish (Danio rerio)
- PMID: 19366995
- PMCID: PMC2724053
- DOI: 10.1194/jlr.M800590-JLR200
Ontogeny and nutritional control of adipogenesis in zebrafish (Danio rerio)
Abstract
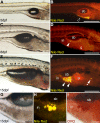
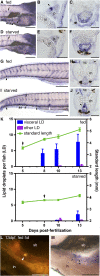
The global obesity epidemic demands an improved understanding of the developmental and environmental factors regulating fat storage. Adipocytes serve as major sites of fat storage and as regulators of energy balance and inflammation. The optical transparency of developing zebrafish provides new opportunities to investigate mechanisms governing adipocyte biology, however zebrafish adipocytes remain uncharacterized. We have developed methods for visualizing zebrafish adipocytes in vivo by labeling neutral lipid droplets with Nile Red. Our results establish that neutral lipid droplets first accumulate in visceral adipocytes during larval stages and increase in number and distribution as zebrafish grow. We show that the cellular anatomy of zebrafish adipocytes is similar to mammalian white adipocytes and identify peroxisome-proliferator activated receptor gamma and fatty acid binding protein 11a as markers of the zebrafish adipocyte lineage. By monitoring adipocyte development prior to neutral lipid deposition, we find that the first visceral preadipocytes appear in association with the pancreas shortly after initiation of exogenous nutrition. Zebrafish reared in the absence of food fail to form visceral preadipocytes, indicating that exogenous nutrition is required for adipocyte development. These results reveal homologies between zebrafish and mammalian adipocytes and establish the zebrafish as a new model for adipocyte research.
Figures




References
-
- Department of Health and Human Services (2001). The Surgeon General's Call to Action to Prevent and Decrease Overweight and Obesity. Public Health Service, Washington, DC
-
- Gesta S., Tseng Y. H., Kahn C. R. 2007. Developmental origin of fat: tracking obesity to its source. Cell. 131: 242–256 - PubMed
-
- Fruhbeck G., Gomez-Ambrosi J., Muruzabal F. J., Burrell M. A. 2001. The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am. J. Physiol. Endocrinol. Metab. 280: E827–E847 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

