Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons
- PMID: 19367261
- PMCID: PMC2835208
- DOI: 10.1038/mt.2009.71
Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons
Abstract
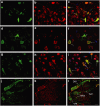
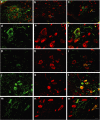
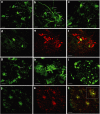
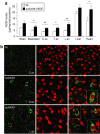
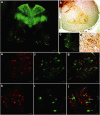
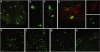
Therapeutic gene delivery to the whole spinal cord is a major challenge for the treatment of motor neuron (MN) diseases. Systemic administration of viral gene vectors would provide an optimal means for the long-term delivery of therapeutic molecules from blood to the spinal cord but this approach is hindered by the presence of the blood-brain barrier (BBB). Here, we describe the first successful study of MN transduction in adult animals following intravenous (i.v.) delivery of self-complementary (sc) AAV9 vectors (up to 28% in mice). Intravenous MN transduction was achieved in adults without pharmacological disruption of the BBB and transgene expression lasted at least 5 months. Importantly, this finding was successfully translated to large animals, with the demonstration of an efficient systemic scAAV9 gene delivery to the neonate and adult cat spinal cord. This new and noninvasive procedure raises the hope of whole spinal cord correction of MN diseases and may lead to the development of new gene therapy protocols in patients.
Figures
References
-
- Monani UR. Spinal muscular atrophy: a deficiency in a ubiquitous protein; a motor neuron-specific disease. Neuron. 2005;48:885–896. - PubMed
-
- Pasinelli P., and , Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci. 2006;7:710–723. - PubMed
-
- Miller RG, Mitchell JD, Lyon M., and , Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) Cochrane Database Syst Rev. 2007;24:CD001447. - PubMed
-
- Azzouz M, Hottinger A, Paterna JC, Zurn AD, Aebischer P., and , Büeler H. Increased motoneuron survival and improved neuromuscular function in transgenic ALS mice after intraspinal injection of an adeno-associated virus encoding Bcl-2. Hum Mol Genet. 2000;9:803–811. - PubMed
-
- Kaspar BK, Lladó J, Sherkat N, Rothstein JD., and , Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

