Patient perspectives with abbreviated versus standard pre-test HIV counseling in the prenatal setting: a randomized-controlled, non-inferiority trial
- PMID: 19367335
- PMCID: PMC2666158
- DOI: 10.1371/journal.pone.0005166
Patient perspectives with abbreviated versus standard pre-test HIV counseling in the prenatal setting: a randomized-controlled, non-inferiority trial
Abstract
Background: In the US, an unacceptably high percentage of pregnant women do not undergo prenatal HIV testing. Previous studies have found increased uptake of prenatal HIV testing with abbreviated pre-test counseling, however little is known about patient decision making, testing satisfaction and knowledge in this setting.
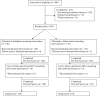
Methodology/findings: A randomized-controlled, non-inferiority trial was conducted from October 2006 through February 2008 at San Francisco General Hospital (SFGH), the public teaching hospital of the City and County of San Francisco. A total of 278 English- and Spanish-speaking pregnant women were randomized to receive either abbreviated or standard nurse-performed HIV test counseling at the initial prenatal visit. Patient decision making experience was compared between abbreviated versus standard HIV counseling strategies among a sample of low-income, urban, ethnically diverse prenatal patients. The primary outcome was the decisional conflict score (DCS) using O'Connor low-literacy scale and secondary outcomes included satisfaction with test decision, basic HIV knowledge and HIV testing uptake. We conducted an intention-to-treat analysis of 278 women--134 (48.2%) in the abbreviated arm (AA) and 144 (51.8%) in the standard arm (SA). There was no significant difference in the proportion of women with low decisional conflict (71.6% in AA vs. 76.4% in SA, p = .37), and the observed mean difference between the groups of 3.88 (95% CI: -0.65, 8.41) did not exceed the non-inferiority margin. HIV testing uptake was very high (97. 8%) and did not differ significantly between the 2 groups (99.3% in AA vs. 96.5% in SA, p = .12). Likewise, there was no difference in satisfaction with testing decision (97.8% in AA vs. 99.3% in SA, p = .36). However, women in AA had significantly lower mean HIV knowledge scores (78.4%) compared to women in SA (83.7%, p<0.01).
Conclusions/significance: This study suggests that streamlining the pre-test counseling process, while associated with slightly lower knowledge, does not compromise patient decision making or satisfaction regarding HIV testing.
Trial registration: ClinicalTrials.gov NCT00503308.
Conflict of interest statement
Figures
References
-
- US Department of Health and Human Services Office of the Inspector General. Reducing Obstetrician Barriers to Offering HIV Testing. Washington, DC: US Department of Health and Human Services; 2002.
-
- (2002) From the Centers for Disease Control and Prevention. Progress toward elimination of perinatal HIV infection–Michigan, 1993–2000. MMWR Morb Mortal Wkly Rep. 51:93–97. - PubMed
-
- (2002) HIV testing among pregnant women–United States and Canada, 1998–2001. MMWR Morb Mortal Wkly Rep. 51:1013–1016. - PubMed
-
- Chou R, Smits AK, Huffman LH, Fu R, Korthuis PT. Prenatal screening for HIV: A review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2005;143:38–54. - PubMed
-
- Phillips KA, Morrison KR, Sonnad SS, Bleecker T. HIV counseling and testing of pregnant women and women of childbearing age by primary care providers: self-reported beliefs and practices. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:174–178. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

