MVA.85A boosting of BCG and an attenuated, phoP deficient M. tuberculosis vaccine both show protective efficacy against tuberculosis in rhesus macaques
- PMID: 19367339
- PMCID: PMC2666807
- DOI: 10.1371/journal.pone.0005264
MVA.85A boosting of BCG and an attenuated, phoP deficient M. tuberculosis vaccine both show protective efficacy against tuberculosis in rhesus macaques
Erratum in
- PLoS One. 2011;6(2). doi:10.1371/annotation/e599dafd-8208-4655-a792-21cb125f7f66
Abstract
Background: Continuous high global tuberculosis (TB) mortality rates and variable vaccine efficacy of Mycobacterium bovis Bacille Calmette-Guérin (BCG) motivate the search for better vaccine regimes. Relevant models are required to downselect the most promising vaccines entering clinical efficacy testing and to identify correlates of protection.
Methods and findings: Here, we evaluated immunogenicity and protection against Mycobacterium tuberculosis in rhesus monkeys with two novel strategies: BCG boosted by modified vaccinia virus Ankara expressing antigen 85A (MVA.85A), and attenuated M. tuberculosis with a disrupted phoP gene (SO2) as a single-dose vaccine. Both strategies were well tolerated, and immunogenic as evidenced by induction of specific IFNgamma responses. Antigen 85A-specific IFNgamma secretion was specifically increased by MVA.85A boosting. Importantly, both MVA.85A and SO2 treatment significantly reduced pathology and chest X-ray scores upon infectious challenge with M. tuberculosis Erdman strain. MVA.85A and SO2 treatment also showed reduced average lung bacterial counts (1.0 and 1.2 log respectively, compared with 0.4 log for BCG) and significant protective effect by reduction in C-reactive protein levels, body weight loss, and decrease of erythrocyte-associated hematologic parameters (MCV, MCH, Hb, Ht) as markers of inflammatory infection, all relative to non-vaccinated controls. Lymphocyte stimulation revealed Ag85A-induced IFNgamma levels post-infection as the strongest immunocorrelate for protection (spearman's rho: -0.60).
Conclusions: Both the BCG/MVA.85A prime-boost regime and the novel live attenuated, phoP deficient TB vaccine candidate SO2 showed significant protective efficacy by various parameters in rhesus macaques. Considering the phylogenetic relationship between macaque and man and the similarity in manifestations of TB disease, these data support further development of these primary and combination TB vaccine candidates.
Conflict of interest statement
Figures
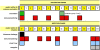





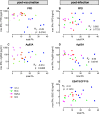
References
-
- Global tuberculosis control: surveillance, planning, financing: WHO report 2008. WHO/HTM/TB/2008.393, at http://www.who.int/tb/publications/2008/en/index.html.
-
- Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339–1345. - PubMed
-
- Skeiky YAW, Sadoff JC. Advances in tuberculosis vaccine strategies. Nat Rev Microbiol. 2006;4:469–476. - PubMed
-
- Baumann S, Nasser Eddine A, Kaufmann SH. Progress in tuberculosis vaccine development. Curr Opin Immunol. 2006;18:438–448. - PubMed
-
- Hussey G, Hawkridge T, Hanekom W. Childhood tuberculosis: old and new vaccines. Paediatr Respir Rev. 2007;8:148–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

