Protein kinase C in porcine retinal arteries and neuroretina following retinal ischemia-reperfusion
- PMID: 19367344
- PMCID: PMC2668768
Protein kinase C in porcine retinal arteries and neuroretina following retinal ischemia-reperfusion
Abstract
Purpose: Identification of the intracellular signal-transduction pathways activated in retinal ischemia may be important in revealing novel pharmacological targets. To date, most studies have focused on identifying neuroprotective agents. The retinal blood vessels are key organs in circulatory failure, and this study was therefore designed to examine the retinal vasculature separately from the neuroretina.
Methods: Retinal ischemia was induced by elevating the intraocular pressure in porcine eyes, followed by 5, 12, or 20 h of reperfusion. Protein kinase C (PKC)alpha, PKCbeta1, and PKCbeta2 mRNA levels, and protein expression were determined using real-time PCR, western blot, and immunofluorescence staining techniques.
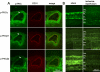
Results: The retinal arteries could easily be dissected free and studied separately from the neuroretina in this porcine model. The PKCalpha, PKCbeta1, and PKCbeta2 mRNA levels tended to be lower in ischemia-reperfused than in sham-operated eyes in both the retinal arteries and the neuroretina. This was most prominent after 5 h, and less pronounced after 12 h and 20 h of reperfusion. Likewise, the protein levels of PKCalpha, PKCbeta1, and PKCbeta2 were slightly lower following ischemia-reperfusion when compared to sham-operated eyes. PKCalpha, PKCbeta1, and PKCbeta2 immunostaining were observed in bipolar cells of the neuroretina and in endothelial cells, and to a low extent in the smooth muscle layer, of the retinal arteries.
Conclusions: Retinal ischemia followed by reperfusion results in lower levels of PKC in both the neuroretina and retinal arteries. New targets for pharmacological treatment may be found by studying the retinal vasculature so as to identify the intracellular signal-transduction pathways involved in the development of injury following retinal circulatory failure.
Figures



References
-
- Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004;23:91–147. - PubMed
-
- Dorrell M, Uusitalo-Jarvinen H, Aguilar E, Friedlander M. Ocular neovascularization: basic mechanisms and therapeutic advances. Surv Ophthalmol. 2007;52(Suppl 1):S3–19. - PubMed
-
- Curtis TM, Scholfield CN. The role of lipids and protein kinase Cs in the pathogenesis of diabetic retinopathy. Diabetes Metab Res Rev. 2004;20:28–43. - PubMed
-
- Comer GM, Ciulla TA. Pharmacotherapy for diabetic retinopathy. Curr Opin Ophthalmol. 2004;15:508–18. - PubMed
-
- Hessellund A, Jeppesen P, Aalkjaer C, Bek T. Characterization of vasomotion in porcine retinal arterioles. Acta Ophthalmol Scand. 2003;81:278–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
