Pharmacotherapeutic modulation of the endocannabinoid signalling system in psychiatric disorders: drug-discovery strategies
- PMID: 19367506
- PMCID: PMC5531754
- DOI: 10.1080/09540260902782778
Pharmacotherapeutic modulation of the endocannabinoid signalling system in psychiatric disorders: drug-discovery strategies
Abstract
Medicinal chemistry has produced small-molecule agents with drug-like character that potently and safely modulate the activity of discrete endocannabinoid system components as potential treatments for medical disorders, including various psychiatric conditions. Two cannabinoid (CB) receptors (CB1 and CB2) currently represent prime endocannabinoid-system therapeutic targets for ligands that either mimic endocannabinoid signalling processes and/or potentiate endocannabinoid-system activity (agonists) or attenuate pathologically heightened endocannabinoid-system transmission (antagonists). Two endocannabinoid deactivating enzymes, fatty acid amide hydrolase (FAAH) and soluble monoacylglycerol lipase (MGL), are increasingly prominent targets for inhibitors that indirectly potentiate endocannabinoid-system signalling. Continued profiling of drug candidates in relevant disease models, identification of additional cannabinoid-related therapeutic targets, and validation of new pharmacological modes of endocannabinoid system modulation will undoubtedly invite further translational efforts in the cannabinoid field for treating psychiatric disorders and other medical conditions.
Conflict of interest statement
Figures
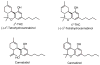
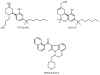
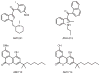
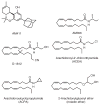
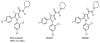
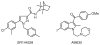
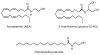
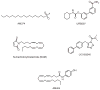
References
-
- Abadji V, Lin S, Taha G, Griffin G, Stevenson LA, Pertwee RG, et al. (R)-methanandamide: A chiral novel anandamide possessing higher potency and metabolic stability. Journal of Medicinal Chemistry. 1994;37:1889–1893. - PubMed
-
- Alexander JP, Cravatt BF. The putative endocannabinoid transport blocker LY2183240 is a potent inhibitor of FAAH and several other brain serine hydrolases. Journal of the American Chemical Society. 2006;128:9699–9704. - PubMed
-
- Anderson BJ. Paracetamol (Acetaminophen): Mechanisms of action. Paediatric Anesthesiology. 2008;18:915–921. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
