The role of hydrogen bonding in tethered polymer layers
- PMID: 19367906
- PMCID: PMC2772075
- DOI: 10.1021/jp8080904
The role of hydrogen bonding in tethered polymer layers
Abstract
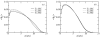
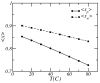
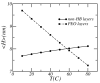
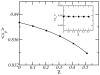
A molecular theory to study the properties of end-tethered polymer layers, in which the polymers have the ability to form hydrogen bonds with water, is presented. The approach combines the ideas of the single-chain mean-field theory to treat tethered layers with the approach of Dormidontova (Macromolecules, 2002, 35, 987.) to include hydrogen bonds. The generalization includes the consideration of position-dependent polymer-water and water-water hydrogen bonds. The theory is applied to model poly(ethylene oxide) (PEO), and the predictions are compared with equivalent polymer layers that do not form hydrogen bonds. It is found that increasing the temperature lowers the solubility of the PEO and results in a collapse of the layer at high enough temperatures. The properties of the layer and their temperature dependence are shown to be the result of the coupling between the conformational entropy of the chains, the ability of the polymer to form hydrogen bonds, and the intermolecular interactions. The structural and thermodynamic properties of the PEO layers, such as the lateral pressure-area isotherms and polymer chemical potentials, are studied as a function of temperature and type of tethering surface. The possibility of phase separation of the PEO layer at high enough temperature is predicted due to the reduced solubility induced by breaking of polymer-water hydrogen bonds. A discussion of the advantages and limitations of the theory, together with how to apply the approach to different hydrogen-bonding polymers, is presented.
Figures





Similar articles
-
Structural behavior of competitive temperature and pH-responsive tethered polymer layers.Soft Matter. 2017 Sep 27;13(37):6322-6331. doi: 10.1039/c7sm01538k. Soft Matter. 2017. PMID: 28905971 Free PMC article.
-
Hydrogen Bond Network Disruption by Hydration Layers in Water Solutions with Salt and Hydrogen-Bonding Polymers (PEO).J Phys Chem B. 2023 Aug 3;127(30):6778-6794. doi: 10.1021/acs.jpcb.3c02505. Epub 2023 Jul 21. J Phys Chem B. 2023. PMID: 37478338
-
Nonfouling poly(ethylene oxide) layers end-tethered to polydopamine.Langmuir. 2012 Oct 9;28(40):14273-83. doi: 10.1021/la3029935. Epub 2012 Sep 28. Langmuir. 2012. PMID: 22989020 Free PMC article.
-
Molecular level studies on interfacial hydration of zwitterionic and other antifouling polymers in situ.Acta Biomater. 2016 Aug;40:6-15. doi: 10.1016/j.actbio.2016.02.030. Epub 2016 Feb 23. Acta Biomater. 2016. PMID: 26923530 Review.
-
Routes to Hydrogen Bonding Chain-End Functionalized Polymers.Macromol Rapid Commun. 2012 Dec 21;33(24):2062-91. doi: 10.1002/marc.201200508. Epub 2012 Nov 7. Macromol Rapid Commun. 2012. PMID: 23136120 Review.
Cited by
-
Structural behavior of competitive temperature and pH-responsive tethered polymer layers.Soft Matter. 2017 Sep 27;13(37):6322-6331. doi: 10.1039/c7sm01538k. Soft Matter. 2017. PMID: 28905971 Free PMC article.
-
Covalent-supramolecular hybrid polymers as muscle-inspired anisotropic actuators.Nat Commun. 2018 Jun 19;9(1):2395. doi: 10.1038/s41467-018-04800-w. Nat Commun. 2018. PMID: 29921928 Free PMC article.
-
Polymers for Disrupting Protein-Protein Interactions: Where Are We and Where Should We Be?Biomacromolecules. 2024 Oct 14;25(10):6229-6249. doi: 10.1021/acs.biomac.4c00850. Epub 2024 Sep 10. Biomacromolecules. 2024. PMID: 39254158 Review.
-
Concentration dependence of lipopolymer self-diffusion in supported bilayer membranes.J R Soc Interface. 2011 Jan 6;8(54):127-43. doi: 10.1098/rsif.2010.0200. Epub 2010 May 26. J R Soc Interface. 2011. PMID: 20504804 Free PMC article.
-
Emerging applications of stimuli-responsive polymer materials.Nat Mater. 2010 Feb;9(2):101-13. doi: 10.1038/nmat2614. Epub 2010 Jan 22. Nat Mater. 2010. PMID: 20094081 Review.
References
-
- Malcolm GN, Rowlinson JS. Trans. Faraday. Soc. 1957;53:921.
-
- Strazielle C. Makromol. Chem. 1968;119:50.
-
- Harris JM, Zalipsky S, editors. Poly(Ethylene Glycol): Chemistry and Biological Applications, American Chemical Society Symposium Series (Am. Chem. Soc., Washington, DC) 1997. (No.680).
-
- Sakai T, Alexandridis P. Nanotechnology. 2005;16:S344. - PubMed
-
- Alexandridis P. Curr. Opin. Colloid Interface Sci. 1997;2:478.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

