Efficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered-dose inhaler in patients with chronic obstructive pulmonary disease: results from a 1-year randomized controlled clinical trial
- PMID: 19368417
- PMCID: PMC3580134
- DOI: 10.2165/00003495-200969050-00004
Efficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered-dose inhaler in patients with chronic obstructive pulmonary disease: results from a 1-year randomized controlled clinical trial
Abstract
Background: Combination therapy with a long-acting bronchodilator and an inhaled corticosteroid (ICS) is recommended in patients with chronic obstructive pulmonary disease (COPD) who have frequent exacerbations. The efficacy and tolerability of the combination of budesonide/formoterol have been demonstrated in patients with COPD when administered via the dry powder inhaler (DPI) in a 1-year study and when administered via the hydrofluoroalkane (HFA) pressurized metered-dose inhaler (pMDI) in a 6-month study.
Objective: This study assessed the long-term efficacy and tolerability of budesonide/formoterol HFA pMDI in patients with moderate to very severe COPD.
Methods: This was a 12-month, randomized, double-blind, double-dummy, parallel-group, active- and placebo-controlled, multicentre study (NCT00206167) of 1, 964 patients aged >or =40 years with moderate to very severe COPD conducted from 2005 to 2007 at 237 sites in the US, Europe and Mexico. After 2 weeks of treatment based on previous therapy (ICSs, short-acting bronchodilators allowed), patients received one of the following treatments twice daily: budesonide/formoterol pMDI 160/4.5 microg x two inhalations (320/9 microg); budesonide/formoterol pMDI 80/4.5 microg x two inhalations (160/9 microg); formoterol DPI 4.5 microg x two inhalations (9 microg); or placebo.
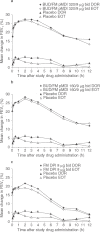
Main outcome measures: The co-primary efficacy variables were pre-dose forced expiratory volume in 1 second (FEV1) and 1-hour post-dose FEV1. .
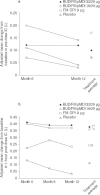
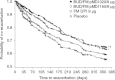
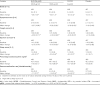
Results: Budesonide/formoterol 320/9 microg demonstrated greater improvements in pre-dose FEV1 versus formoterol (p = 0.008), and both budesonide/formoterol doses demonstrated greater improvements in 1-hour post-dose FEV1 versus placebo (p < 0.001). The rate of COPD exacerbations was lower in both budesonide/formoterol groups compared with formoterol and placebo (p <or= 0.004). Both budesonide/formoterol doses were more effective than placebo (p <or= 0.006) for controlling dyspnoea and improving health status (St George's Respiratory Questionnaire). All treatments were generally well tolerated. The incidence of pneumonia was not different for active (3.4-4.0%) and placebo (5.0%) groups.
Conclusions: Budesonide/formoterol pMDI (320/9 microg and 160/9 microg) improved pulmonary function and reduced symptoms and exacerbations over 1 year in patients with moderate to very severe COPD. Only budesonide/formoterol pMDI 320/9 microg demonstrated greater efficacy for both co-primary variables compared with formoterol DPI 9 microg. Both budesonide/formoterol pMDI dosages were well tolerated relative to formoterol and placebo.
Figures







References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: updated 2007 [online]. Available from URL: http://www.goldcopd.com. [Accessed 2008 Feb 12]
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

