The structural role of the zinc ion can be dispensable in prokaryotic zinc-finger domains
- PMID: 19369210
- PMCID: PMC2678482
- DOI: 10.1073/pnas.0810003106
The structural role of the zinc ion can be dispensable in prokaryotic zinc-finger domains
Abstract
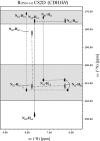
The recent characterization of the prokaryotic Cys(2)His(2) zinc-finger domain, identified in Ros protein from Agrobacterium tumefaciens, has demonstrated that, although possessing a similar zinc coordination sphere, this domain is structurally very different from its eukaryotic counterpart. A search in the databases has identified approximately 300 homologues with a high sequence identity to the Ros protein, including the amino acids that form the extensive hydrophobic core in Ros. Surprisingly, the Cys(2)His(2) zinc coordination sphere is generally poorly conserved in the Ros homologues, raising the question of whether the zinc ion is always preserved in these proteins. Here, we present a functional and structural study of a point mutant of Ros protein, Ros(56-142)C82D, in which the second coordinating cysteine is replaced by an aspartate, 5 previously-uncharacterized representative Ros homologues from Mesorhizobium loti, and 2 mutants of the homologues. Our results indicate that the prokaryotic zinc-finger domain, which in Ros protein tetrahedrally coordinates Zn(II) through the typical Cys(2)His(2) coordination, in Ros homologues can either exploit a CysAspHis(2) coordination sphere, previously never described in DNA binding zinc finger domains to our knowledge, or lose the metal, while still preserving the DNA-binding activity. We demonstrate that this class of prokaryotic zinc-finger domains is structurally very adaptable, and surprisingly single mutations can transform a zinc-binding domain into a nonzinc-binding domain and vice versa, without affecting the DNA-binding ability. In light of our findings an evolutionary link between the prokaryotic and eukaryotic zinc-finger domains, based on bacteria-to-eukaryota horizontal gene transfer, is discussed.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Klug A, Schwabe JW. Protein motifs 5. Zinc fingers. FASEB J. 1995;9:597–604. - PubMed
-
- Laithy JH, Lee BM, Wright PE. Zinc finger proteins: New insights into structural and functional diversity. Curr Opin Struct Biol. 2001;11:39–46. - PubMed
-
- Gamsjaeger R, et al. Sticky fingers: Zinc fingers as protein-recognition motifs. Trends Biochem Sc. 2007;32:63–70. - PubMed
-
- Brayer KJ, Segal DJ. The protein-binding potential of C2H2 zinc finger domains. Cell Biochem Biophys. 2008;50:111–131. - PubMed
-
- Brown RS. Zinc finger proteins: Getting a grip on RNA. Curr Opin Struct Biol. 2005;15:94–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

