Caveolin-1 directly interacts with UT-A1 urea transporter: the role of caveolae/lipid rafts in UT-A1 regulation at the cell membrane
- PMID: 19369293
- PMCID: PMC2692441
- DOI: 10.1152/ajprenal.00068.2009
Caveolin-1 directly interacts with UT-A1 urea transporter: the role of caveolae/lipid rafts in UT-A1 regulation at the cell membrane
Abstract
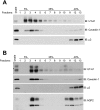
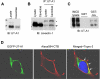
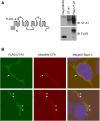
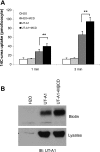
The cell plasma membrane contains specialized microdomains called lipid rafts which contain high amounts of sphingolipids and cholesterol. Lipid rafts are involved in a number of membrane protein functions. The urea transporter UT-A1, located in the kidney inner medullary collecting duct (IMCD), is important for urine concentrating ability. In this study, we investigated the possible role of lipid rafts in UT-A1 membrane regulation. Using sucrose gradient cell fractionation, we demonstrated that UT-A1 is concentrated in the caveolae-rich fraction both in stably expressing UT-A1 HEK293 cells and in freshly isolated kidney IMCD suspensions. In these gradients, UT-A1 at the cell plasma membrane is codistributed with caveolin-1, a major component of caveolae. The colocalization of UT-A1 in lipid rafts/caveolae was further confirmed in isolated caveolae from UT-A1-HEK293 cells. The direct association of UT-A1 and caveolin-1 was identified by immunoprecipitation and GST pull-down assay. Examination of internalized UT-A1 in pEGFP-UT-A1 transfected HEK293 cells fluorescent overlap with labeled cholera toxin subunit B, a marker of the caveolae-mediated endocytosis pathway. Disruption of lipid rafts by methyl-beta-cyclodextrin or knocking down caveolin-1 by small-interference RNA resulted in UT-A1 cell membrane accumulation. Functionally, overexpression of caveolin-1 in oocytes decreased UT-A1 urea transport activity and UT-A1 cell surface expression. Our results indicate that lipid rafts/caveolae participate in UT-A1 membrane regulation and this effect is mediated via a direct interaction of caveolin-1 with UT-A1.
Figures


References
-
- Bradford AD, Terris JM, Ecelbarger CA, Klein JD, Sands JM, Chou CL, Knepper MA. 97- And 117-kDa forms of collecting duct urea transporter UT-A1 are due to different states of glycosylation. Am J Physiol Renal Physiol 281: F133–F143, 2001. - PubMed
-
- Cha SK, Wu T, Huang CL. Protein kinase C inhibits caveolae-mediated endocytosis of TRPV5. Am J Physiol Renal Physiol 294: F1212–F1221, 2008. - PubMed
-
- Chen G, Fröhlich O, Yang Y, Klein JD, Sands JM. Loss of N-linked glycosylation reduces urea transporter UT-A1 response to vasopressin. J Biol Chem 281: 27436–27442, 2006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

