Plant geminivirus rep protein induces rereplication in fission yeast
- PMID: 19369323
- PMCID: PMC2698514
- DOI: 10.1128/JVI.02491-08
Plant geminivirus rep protein induces rereplication in fission yeast
Abstract
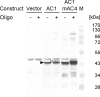
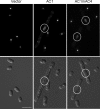
The replication-associated protein (Rep) of geminiviruses, single-stranded DNA viruses of higher plants, is essential for virus replication. Since these viruses do not encode their own polymerases, Rep induces differentiated plant cells to reenter the cell cycle by interacting with the plant homologues of retinoblastoma proteins in order to activate the host DNA synthesis machinery. We have used fission yeast (Schizosaccharomyces pombe) as a model organism to analyze the impact of ectopically expressed African cassava mosaic virus Rep protein on the cell division cycle in closer detail. Upon expression, Rep showed its characteristic DNA cleavage activity, and about 10% of the cells exhibited morphological changes. They were elongated threefold, on average, and possessed a single but enlarged and less compact nucleus in comparison to noninduced or vector-only control cells. Flow cytometry of Rep-expressing cultures revealed a distinct subpopulation of Rep protein-containing cells with aberrant morphology. The other 90% of the cells were indistinguishable from control cells, and no Rep was detectable. Rep-expressing cells exhibited DNA contents beyond 2C, indicating ongoing replication without intervening mitosis. Because a second open reading frame (ORF), AC4, is present within the Rep gene, the role of AC4 was examined by destroying its start codon within the AC1 ORF. The results confirmed that Rep is necessary and sufficient to induce rereplication in fission yeast. The unique potential of this well-investigated model for dissecting the cell cycle control by geminiviral proteins is discussed.
Figures
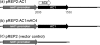


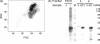
References
-
- Aberle, H. J., M. L. Rütz, M. Karayavuz, S. Frischmuth, C. Wege, D. Hülser, and H. Jeske. 2002. Localizing BC1 movement proteins of Abutilon mosaic geminivirus in yeasts by subcellular fractionation and freeze-fracture immunolabelling. Arch. Virol. 147103-107. - PubMed
-
- Alfa, C., P. Fantes, J. Hyams, M. McLeod, and E. Warbrick. 1993. Experiments with fission yeast: a laboratory course manual. Cold Spring Harbor Press, Cold Spring Harbor, NY.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

