Intracellular localization of hepatitis delta virus proteins in the presence and absence of viral RNA accumulation
- PMID: 19369324
- PMCID: PMC2698582
- DOI: 10.1128/JVI.00008-09
Intracellular localization of hepatitis delta virus proteins in the presence and absence of viral RNA accumulation
Abstract
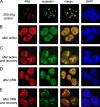
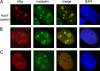
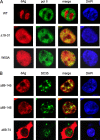
Hepatitis delta virus (HDV) encodes one protein, hepatitis delta antigen (deltaAg), a 195-amino-acid RNA binding protein essential for the accumulation of HDV RNA-directed RNA transcripts. It has been accepted that deltaAg localizes predominantly to the nucleolus in the absence of HDV genome replication while in the presence of replication, deltaAg facilitates HDV RNA transport to the nucleoplasm and helps redirect host RNA polymerase II (Pol II) to achieve transcription and accumulation of processed HDV RNA species. This study used immunostaining and confocal microscopy to evaluate factors controlling the localization of deltaAg in the presence and absence of replicating and nonreplicating HDV RNAs. When deltaAg was expressed in the absence of full-length HDV RNAs, it colocalized with nucleolin, a predominant nucleolar protein. With time, or more quickly after induced cell stress, there was a redistribution of both deltaAg and nucleolin to the nucleoplasm. Following expression of nonreplicating HDV RNAs, deltaAg moved to the nucleoplasm, but nucleolin was unchanged. When deltaAg was expressed along with replicating HDV RNA, it was found predominantly in the nucleoplasm along with Pol II. This localization was insensitive to inhibitors of HDV replication, suggesting that the majority of deltaAg in the nucleoplasm reflects ribonucleoprotein accumulation rather than ongoing transcription. An additional approach was to reevaluate several forms of deltaAg altered at specific locations considered to be essential for protein function. These studies provide evidence that deltaAg does not interact directly with either Pol II or nucleolin and that forms of deltaAg which support replication are also capable of prior nucleolar transit.
Figures



Similar articles
-
Redistribution of the delta antigens in cells replicating the genome of hepatitis delta virus.J Virol. 1996 Nov;70(11):8064-70. doi: 10.1128/JVI.70.11.8064-8070.1996. J Virol. 1996. PMID: 8892931 Free PMC article.
-
Parameters of human hepatitis delta virus genome replication: the quantity, quality, and intracellular distribution of viral proteins and RNA.J Virol. 2002 Apr;76(8):3709-19. doi: 10.1128/jvi.76.8.3709-3719.2002. J Virol. 2002. PMID: 11907210 Free PMC article.
-
Transcription of hepatitis delta virus RNA by RNA polymerase II.J Virol. 2008 Feb;82(3):1118-27. doi: 10.1128/JVI.01758-07. Epub 2007 Nov 21. J Virol. 2008. PMID: 18032511 Free PMC article.
-
Chapter 3. Replication of the hepatitis delta virus RNA genome.Adv Virus Res. 2009;74:103-21. doi: 10.1016/S0065-3527(09)74003-5. Adv Virus Res. 2009. PMID: 19698896 Review.
-
The molecular biology of hepatitis delta virus.Annu Rev Biochem. 1995;64:259-86. doi: 10.1146/annurev.bi.64.070195.001355. Annu Rev Biochem. 1995. PMID: 7574482 Review.
Cited by
-
Hepatitis Delta Antigen Regulates mRNA and Antigenome RNA Levels during Hepatitis Delta Virus Replication.J Virol. 2019 Apr 3;93(8):e01989-18. doi: 10.1128/JVI.01989-18. Print 2019 Apr 15. J Virol. 2019. PMID: 30728256 Free PMC article.
-
Hepatitis delta antigen requires a flexible quasi-double-stranded RNA structure to bind and condense hepatitis delta virus RNA in a ribonucleoprotein complex.J Virol. 2014 Jul;88(13):7402-11. doi: 10.1128/JVI.00443-14. Epub 2014 Apr 16. J Virol. 2014. PMID: 24741096 Free PMC article.
-
Intrinsic disorder and oligomerization of the hepatitis delta virus antigen.Virology. 2010 Nov 25;407(2):333-40. doi: 10.1016/j.virol.2010.08.019. Epub 2010 Sep 19. Virology. 2010. PMID: 20855099 Free PMC article.
-
Intrinsically disordered proteins of viruses: Involvement in the mechanism of cell regulation and pathogenesis.Prog Mol Biol Transl Sci. 2020;174:1-78. doi: 10.1016/bs.pmbts.2020.03.001. Epub 2020 Apr 2. Prog Mol Biol Transl Sci. 2020. PMID: 32828463 Free PMC article. Review.
-
Prokaryotic Expression, Purification and Immunogenicity in Rabbits of the Small Antigen of Hepatitis Delta Virus.Int J Mol Sci. 2016 Oct 20;17(10):1721. doi: 10.3390/ijms17101721. Int J Mol Sci. 2016. PMID: 27775592 Free PMC article.
References
-
- Alves, C., N. Freitas, and C. Cunha. 2008. Characterization of the nuclear localization signal of the hepatitis delta virus antigen. Virology 37012-21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

