Dissecting the functional domains of a nonenveloped virus membrane penetration peptide
- PMID: 19369344
- PMCID: PMC2698515
- DOI: 10.1128/JVI.02299-08
Dissecting the functional domains of a nonenveloped virus membrane penetration peptide
Abstract
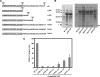
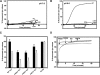
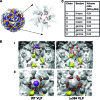
Recent studies have established that several nonenveloped viruses utilize virus-encoded lytic peptides for host membrane disruption. We investigated this mechanism with the "gamma" peptide of the insect virus Flock House virus (FHV). We demonstrate that the C terminus of gamma is essential for membrane disruption in vitro and the rescue of immature virus infectivity in vivo, and the amphipathic N terminus of gamma alone is not sufficient. We also show that deletion of the C-terminal domain disrupts icosahedral ordering of the amphipathic helices of gamma in the virus. Our results have broad implications for understanding membrane lysis during nonenveloped virus entry.
Figures
Similar articles
-
Rescue of maturation-defective flock house virus infectivity with noninfectious, mature, viruslike particles.J Virol. 2008 Feb;82(4):2025-7. doi: 10.1128/JVI.02278-07. Epub 2007 Dec 12. J Virol. 2008. PMID: 18077727 Free PMC article.
-
Structure and function of a genetically engineered mimic of a nonenveloped virus entry intermediate.J Virol. 2010 May;84(9):4737-46. doi: 10.1128/JVI.02670-09. Epub 2010 Feb 17. J Virol. 2010. PMID: 20164221 Free PMC article.
-
Non-Enveloped Virus Entry: Structural Determinants and Mechanism of Functioning of a Viral Lytic Peptide.J Mol Biol. 2016 Aug 28;428(17):3540-56. doi: 10.1016/j.jmb.2016.06.006. Epub 2016 Jun 16. J Mol Biol. 2016. PMID: 27320388
-
Flock house virus: a model system for understanding non-enveloped virus entry and membrane penetration.Curr Top Microbiol Immunol. 2010;343:1-22. doi: 10.1007/82_2010_35. Curr Top Microbiol Immunol. 2010. PMID: 20407886 Review.
-
Breach: Host Membrane Penetration and Entry by Nonenveloped Viruses.Trends Microbiol. 2018 Jun;26(6):525-537. doi: 10.1016/j.tim.2017.09.010. Epub 2017 Oct 25. Trends Microbiol. 2018. PMID: 29079499 Review.
Cited by
-
Strategies for antiviral screening targeting early steps of virus infection.Virol Sin. 2010 Aug;25(4):281-93. doi: 10.1007/s12250-010-3135-z. Epub 2010 Jul 28. Virol Sin. 2010. PMID: 20960301 Free PMC article. Review.
-
Atomistic dynamics of a viral infection process: Release of membrane lytic peptides from a non-enveloped virus.Sci Adv. 2021 Apr 14;7(16):eabe1761. doi: 10.1126/sciadv.abe1761. Print 2021 Apr. Sci Adv. 2021. PMID: 33853772 Free PMC article.
-
Folding a viral peptide in different membrane environments: pathway and sampling analyses.J Biol Phys. 2018 Jun;44(2):195-209. doi: 10.1007/s10867-018-9490-y. Epub 2018 Apr 11. J Biol Phys. 2018. PMID: 29644513 Free PMC article.
-
Influence of membrane composition on the binding and folding of a membrane lytic peptide from the non-enveloped flock house virus.Biochim Biophys Acta Biomembr. 2017 Jul;1859(7):1190-1199. doi: 10.1016/j.bbamem.2017.04.002. Epub 2017 Apr 7. Biochim Biophys Acta Biomembr. 2017. PMID: 28395954 Free PMC article.
-
Evolution in action: N and C termini of subunits in related T = 4 viruses exchange roles as molecular switches.Structure. 2010 Jun 9;18(6):700-9. doi: 10.1016/j.str.2010.03.010. Structure. 2010. PMID: 20541507 Free PMC article.
References
-
- Banerjee, M., and J. E. Johnson. 2008. Activation, exposure and penetration of virally encoded, membrane-active polypeptides during non-enveloped virus entry. Curr. Protein Pept Sci. 916-27. - PubMed
-
- Bong, D. T., C. Steinem, A. Janshoff, J. E. Johnson, and M. Reza Ghadiri. 1999. A highly membrane-active peptide in Flock House virus: implications for the mechanism of nodavirus infection. Chem. Biol. 6473-481. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

