Notch signaling controls liver development by regulating biliary differentiation
- PMID: 19369401
- PMCID: PMC2673761
- DOI: 10.1242/dev.029140
Notch signaling controls liver development by regulating biliary differentiation
Abstract
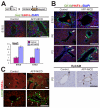
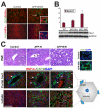
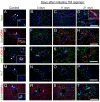
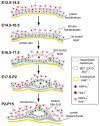
In the mammalian liver, bile is transported to the intestine through an intricate network of bile ducts. Notch signaling is required for normal duct formation, but its mode of action has been unclear. Here, we show in mice that bile ducts arise through a novel mechanism of tubulogenesis involving sequential radial differentiation. Notch signaling is activated in a subset of liver progenitor cells fated to become ductal cells, and pathway activation is necessary for biliary fate. Notch signals are also required for bile duct morphogenesis, and activation of Notch signaling in the hepatic lobule promotes ectopic biliary differentiation and tubule formation in a dose-dependent manner. Remarkably, activation of Notch signaling in postnatal hepatocytes causes them to adopt a biliary fate through a process of reprogramming that recapitulates normal bile duct development. These results reconcile previous conflicting reports about the role of Notch during liver development and suggest that Notch acts by coordinating biliary differentiation and morphogenesis.
Figures
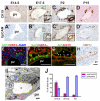
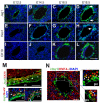
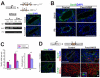
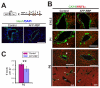

References
-
- Ader, T., Norel, R., Levoci, L. and Rogler, L. E. (2006). Transcriptional profiling implicates TGFbeta/BMP and Notch signaling pathways in ductular differentiation of fetal murine hepatoblasts. Mech. Dev. 123, 177-194. - PubMed
-
- Alagille, D., Estrada, A., Hadchouel, M., Gautier, M., Odievre, M. and Dommergues, J. P. (1987). Syndromic paucity of interlobular bile ducts (Alagille syndrome or arteriohepatic dysplasia): review of 80 cases. J. Pediatr. 110, 195-200. - PubMed
-
- Antoniou, A., Raynaud, P., Cordi, S., Zong, Y., Tronche, F., Stanger, B. Z., Jacquemin, P., Pierreux, C. E., Clotman, F. and Lemaigre, F. P. (2009). Intrahepatic bile ducts develop according to a new mode of tubulogenesis regulated by the transcription factor SOX9. Gastroenterology (in press). - PMC - PubMed
-
- Bolos, V., Grego-Bessa, J. and de la Pompa, J. L. (2007). Notch signaling in development and cancer. Endocr. Rev. 28, 339-363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

