Drosophila motor axons recognize and follow a Sidestep-labeled substrate pathway to reach their target fields
- PMID: 19369411
- PMCID: PMC2682951
- DOI: 10.1101/gad.520509
Drosophila motor axons recognize and follow a Sidestep-labeled substrate pathway to reach their target fields
Abstract
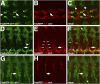
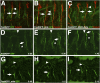
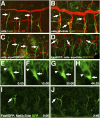
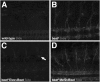
During development of the Drosophila nervous system, migrating motor axons contact and interact with different cell types before reaching their peripheral muscle fields. The axonal attractant Sidestep (Side) is expressed in most of these intermediate targets. Here, we show that motor axons recognize and follow Side-expressing cell surfaces from the ventral nerve cord to their target region. Contact of motor axons with Side-expressing cells induces the down-regulation of Side. In the absence of Side, the interaction with intermediate targets is lost. Misexpression of Side in side mutants strongly attracts motor axons to ectopic sites. We provide evidence that, on motor axons, Beaten path Ia (Beat) functions as a receptor or part of a receptor complex for Side. In beat mutants, motor axons no longer recognize Side-expressing cell surfaces. Furthermore, Beat interacts with Side both genetically and biochemically. These results suggest that the tracing of Side-labeled cell surfaces by Beat-expressing growth cones is a major principle of motor axon guidance in Drosophila.
Figures


Comment in
-
Choosing the road less traveled by: a ligand-receptor system that controls target recognition by Drosophila motor axons.Genes Dev. 2009 May 1;23(9):1042-5. doi: 10.1101/gad.1803009. Epub 2009 Apr 15. Genes Dev. 2009. PMID: 19369412
References
-
- Aberle H., Haghighi A.P., Fetter R.D., McCabe B.D., Magalhaes T.R., Goodman C.S. wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron. 2002;33:545–558. - PubMed
-
- Araujo S.J., Tear G. Axon guidance mechanisms and molecules: Lessons from invertebrates. Nat. Rev. Neurosci. 2003;4:910–922. - PubMed
-
- Bate C.M. Pioneer neurones in an insect embryo. Nature. 1976;260:54–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
