Functional overlap of microtubule assembly factors in chromatin-promoted spindle assembly
- PMID: 19369413
- PMCID: PMC2688555
- DOI: 10.1091/mbc.e09-01-0043
Functional overlap of microtubule assembly factors in chromatin-promoted spindle assembly
Abstract
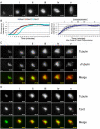
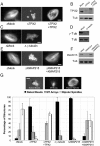
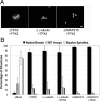
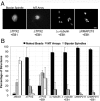
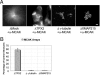
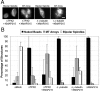
Distinct pathways from centrosomes and chromatin are thought to contribute in parallel to microtubule nucleation and stabilization during animal cell mitotic spindle assembly, but their full mechanisms are not known. We investigated the function of three proposed nucleation/stabilization factors, TPX2, gamma-tubulin and XMAP215, in chromatin-promoted assembly of anastral spindles in Xenopus laevis egg extract. In addition to conventional depletion-add back experiments, we tested whether factors could substitute for each other, indicative of functional redundancy. All three factors were required for microtubule polymerization and bipolar spindle assembly around chromatin beads. Depletion of TPX2 was partially rescued by the addition of excess XMAP215 or EB1, or inhibiting MCAK (a Kinesin-13). Depletion of either gamma-tubulin or XMAP215 was partially rescued by adding back XMAP215, but not by adding any of the other factors. These data reveal functional redundancy between specific assembly factors in the chromatin pathway, suggesting individual proteins or pathways commonly viewed to be essential may not have entirely unique functions.
Figures
References
-
- Basto R., Lau J., Vinogradova T., Gardiol A., Woods C. G., Khodjakov A., Raff J. W. Flies without centrioles. Cell. 2006;125:1375–1386. - PubMed
-
- Basto R., Pines J. The centrosome opens the way to mitosis. Dev. Cell. 2007;12:475–477. - PubMed
-
- Carazo-Salas R. E., Gruss O. J., Mattaj I. W., Karsenti E. Ran-GTP coordinates regulation of microtubule nucleation and dynamics during mitotic-spindle assembly. Nat. Cell Biol. 2001;3:228–234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

