LKB1 catalytic activity contributes to estrogen receptor alpha signaling
- PMID: 19369417
- PMCID: PMC2688557
- DOI: 10.1091/mbc.e08-11-1138
LKB1 catalytic activity contributes to estrogen receptor alpha signaling
Abstract
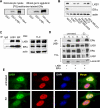
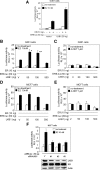
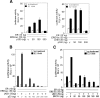
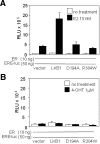
The tumor suppressor serine-threonine kinase LKB1 is mutated in Peutz-Jeghers syndrome (PJS) and in epithelial cancers, including hormone-sensitive organs such as breast, ovaries, testes, and prostate. Clinical studies in breast cancer patients show low LKB1 expression is related to poor prognosis, whereas in PJS, the risk of breast cancer is similar to the risk from germline mutations in breast cancer (BRCA) 1/BRCA2. In this study, we investigate the role of LKB1 in estrogen receptor alpha (ERalpha) signaling. We demonstrate for the first time that LKB1 binds to ERalpha in the cell nucleus in which it is recruited to the promoter of ERalpha-responsive genes. Furthermore, LKB1 catalytic activity enhances ERalpha transactivation compared with LKB1 catalytically deficient mutants. The significance of our discovery is that we demonstrate for the first time a novel functional link between LKB1 and ERalpha. Our discovery places LKB1 in a coactivator role for ERalpha signaling, broadening the scientific scope of this tumor suppressor kinase and laying the groundwork for the use of LKB1 as a target for the development of new therapies against breast cancer.
Figures




Similar articles
-
Liver kinase B1 expression (LKB1) is repressed by estrogen receptor alpha (ERα) in MCF-7 human breast cancer cells.Biochem Biophys Res Commun. 2012 Jan 20;417(3):1063-8. doi: 10.1016/j.bbrc.2011.12.096. Epub 2011 Dec 26. Biochem Biophys Res Commun. 2012. PMID: 22226967
-
Stimulatory cross-talk between NFAT3 and estrogen receptor in breast cancer cells.J Biol Chem. 2005 Dec 30;280(52):43188-97. doi: 10.1074/jbc.M506598200. Epub 2005 Oct 11. J Biol Chem. 2005. PMID: 16219765
-
The cell fate determination factor DACH1 is expressed in estrogen receptor-alpha-positive breast cancer and represses estrogen receptor-alpha signaling.Cancer Res. 2009 Jul 15;69(14):5752-60. doi: 10.1158/0008-5472.CAN-08-3992. Cancer Res. 2009. PMID: 19605405 Free PMC article.
-
The molecular mechanisms that underlie the tumor suppressor function of LKB1.Acta Biochim Biophys Sin (Shanghai). 2009 Feb;41(2):97-107. doi: 10.1093/abbs/gmn011. Acta Biochim Biophys Sin (Shanghai). 2009. PMID: 19204826 Review.
-
Targeting the LKB1 tumor suppressor.Curr Drug Targets. 2014 Jan;15(1):32-52. doi: 10.2174/1389450114666140106095811. Curr Drug Targets. 2014. PMID: 24387336 Free PMC article. Review.
Cited by
-
Genomic-Epidemiologic Evidence That Estrogens Promote Breast Cancer Development.Cancer Epidemiol Biomarkers Prev. 2018 Aug;27(8):899-907. doi: 10.1158/1055-9965.EPI-17-1174. Epub 2018 May 22. Cancer Epidemiol Biomarkers Prev. 2018. PMID: 29789325 Free PMC article.
-
Omega-3 polyunsaturated fatty acid promotes the inhibition of glycolytic enzymes and mTOR signaling by regulating the tumor suppressor LKB1.Cancer Biol Ther. 2013 Nov;14(11):1050-8. doi: 10.4161/cbt.26206. Epub 2013 Sep 6. Cancer Biol Ther. 2013. PMID: 24025358 Free PMC article.
-
Mechanisms of environmental chemicals that enable the cancer hallmark of evasion of growth suppression.Carcinogenesis. 2015 Jun;36 Suppl 1(Suppl 1):S2-18. doi: 10.1093/carcin/bgv028. Carcinogenesis. 2015. PMID: 26106139 Free PMC article. Review.
-
Liver Kinase B1-A Potential Therapeutic Target in Hormone-Sensitive Breast Cancer in Older Women.Cancers (Basel). 2019 Jan 28;11(2):149. doi: 10.3390/cancers11020149. Cancers (Basel). 2019. PMID: 30696074 Free PMC article.
-
The LKB1 complex-AMPK pathway: the tree that hides the forest.Fam Cancer. 2011 Sep;10(3):415-24. doi: 10.1007/s10689-011-9457-7. Fam Cancer. 2011. PMID: 21656073 Free PMC article. Review.
References
-
- Augereau P., Miralles F., Cavailles V., Gaudelet C., Parker M., Rochefort H. Characterization of the proximal estrogen-responsive element of human cathepsin D gene. Mol. Endocrinol. 1994;8:693–703. - PubMed
-
- Barnes D., Gillett C. Cyclin D1 in breast cancer. Breast Cancer Res. Treat. 1998;52:1–15. - PubMed
-
- Batsche E., Desroches J., Bilodeau S., Gauthier Y., Drouin J. Rb enhances p160/SRC coactivator-dependent activity of nuclear receptors and hormone responsiveness. J. Biol. Chem. 2005;280:19746–19756. - PubMed
-
- Bignell G. R., Barfoot R., Seal S., Collins N., Warren W., Stratton M. R. Low frequency of somatic mutations in the LKB1/Peutz-Jeghers syndrome gene in sporadic breast cancer. Cancer Res. 1998;58:1384–1386. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

