Identification of the neuroblastoma-amplified gene product as a component of the syntaxin 18 complex implicated in Golgi-to-endoplasmic reticulum retrograde transport
- PMID: 19369418
- PMCID: PMC2688544
- DOI: 10.1091/mbc.e08-11-1104
Identification of the neuroblastoma-amplified gene product as a component of the syntaxin 18 complex implicated in Golgi-to-endoplasmic reticulum retrograde transport
Abstract
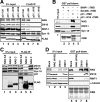
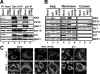
Syntaxin 18, a soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptor (SNARE) protein implicated in endoplasmic reticulum (ER) membrane fusion, forms a complex with other SNAREs (BNIP1, p31, and Sec22b) and several peripheral membrane components (Sly1, ZW10, and RINT-1). In the present study, we showed that a peripheral membrane protein encoded by the neuroblastoma-amplified gene (NAG) is a subunit of the syntaxin 18 complex. NAG encodes a protein of 2371 amino acids, which exhibits weak similarity to yeast Dsl3p/Sec39p, an 82-kDa component of the complex containing the yeast syntaxin 18 orthologue Ufe1p. Under conditions favoring SNARE complex disassembly, NAG was released from syntaxin 18 but remained in a p31-ZW10-RINT-1 subcomplex. Binding studies showed that the extreme N-terminal region of p31 is responsible for the interaction with NAG and that the N- and the C-terminal regions of NAG interact with p31 and ZW10-RINT-1, respectively. Knockdown of NAG resulted in a reduction in the expression of p31, confirming their intimate relationship. NAG depletion did not substantially affect Golgi morphology and protein export from the ER, but it caused redistribution of Golgi recycling proteins accompanied by a defect in protein glycosylation. These results together suggest that NAG links between p31 and ZW10-RINT-1 and is involved in Golgi-to-ER transport.
Figures







References
-
- Andag U., Neumann T., Schmitt H. D. The coatomer-interacting protein Dsl1p is required for Golgi-to-endoplasmic reticulum retrieval in yeast. J. Biol. Chem. 2001;276:39150–39160. - PubMed
-
- Andag U., Schmitt H. D. Dsl1p, an essential component of the Golgi-endoplasmic reticulum retrieval system in yeast, uses the same sequence motif to interact with different subunits of the COPI vesicle coat. J. Biol. Chem. 2003;278:51722–51734. - PubMed
-
- Aoki T., Kojima M., Tani K., Tagaya M. Sec22b-dependent assembly of endoplasmic reticulum Q-SNARE proteins. Biochem. J. 2008;410:93–100. - PubMed
-
- Appenzeller-Herzog C., Hauri H. P. The ER-Golgi intermediate compartment (ERGIC): in search of its identity and function. J. Cell Sci. 2006;119:2173–2183. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

